| Structure | Name/CAS No. | Articles |
|---|---|---|
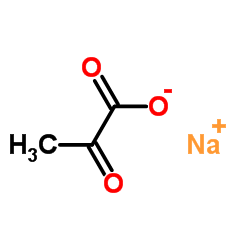 |
Sodium 2-oxopropanoate
CAS:113-24-6 |
|
 |
Acetonitrile
CAS:75-05-8 |
|
 |
trifluoroacetic acid
CAS:76-05-1 |
|
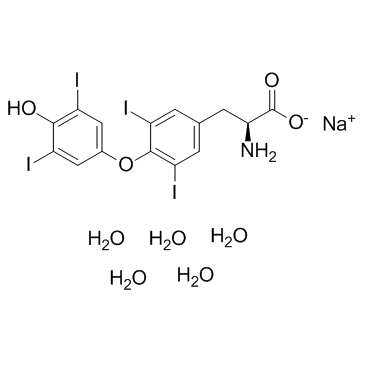 |
Sodium levothyroxine pentahydrate
CAS:6106-07-6 |
|
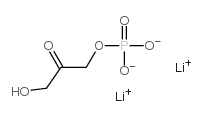 |
Dihydroxyacetone phosphate dilithium salt
CAS:102783-56-2 |
|
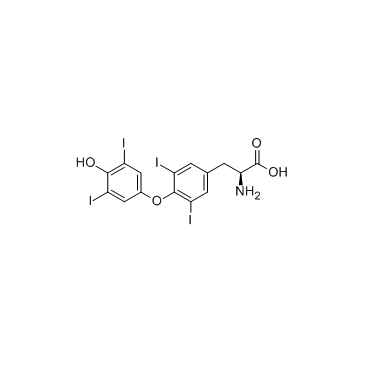 |
L-thyroxine
CAS:51-48-9 |