| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Diethyl ether
CAS:60-29-7 |
|
 |
Acetonitrile
CAS:75-05-8 |
|
 |
trifluoroacetic acid
CAS:76-05-1 |
|
 |
Water
CAS:7732-18-5 |
|
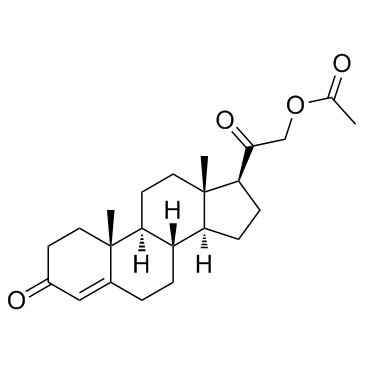 |
Deoxycorticosterone acetate
CAS:56-47-3 |