| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Diethyl ether
CAS:60-29-7 |
|
 |
Dichloromethane
CAS:75-09-2 |
|
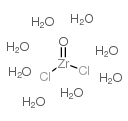 |
Zirconyl chloride octahydrate
CAS:13520-92-8 |
|
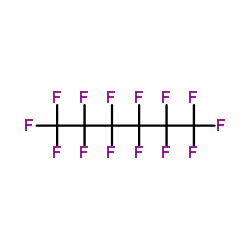 |
Perfluorohexane
CAS:355-42-0 |