| Structure | Name/CAS No. | Articles |
|---|---|---|
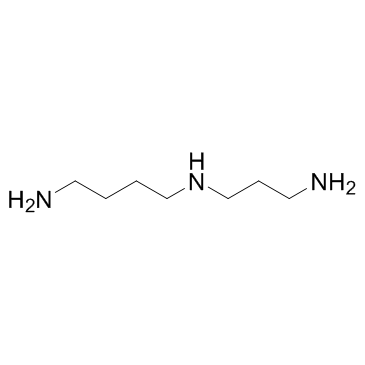 |
spermidine
CAS:124-20-9 |
|
 |
Phenol
CAS:108-95-2 |
|
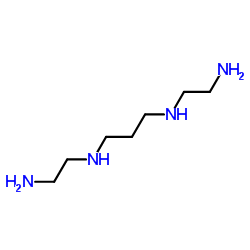 |
n,n'-bis(2-aminoethyl)propan-1,3-diamin
CAS:4741-99-5 |
|
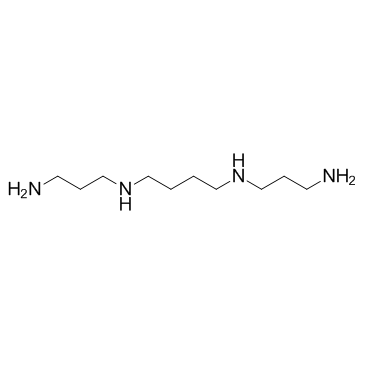 |
Spermine
CAS:71-44-3 |
|
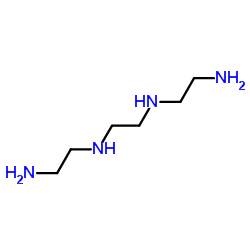 |
Triethylenetetramine
CAS:112-24-3 |
|
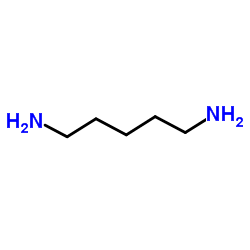 |
Cadaverine
CAS:462-94-2 |
|
 |
1,6-Hexanediamine
CAS:124-09-4 |
|
 |
1,1,4,7,10,10-Hexamethyltriethylenetetramine
CAS:3083-10-1 |