| Structure | Name/CAS No. | Articles |
|---|---|---|
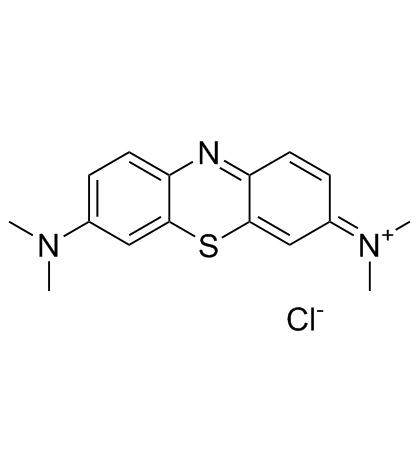 |
Methylene Blue
CAS:61-73-4 |
|
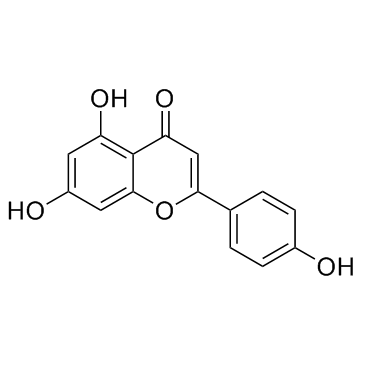 |
Apigenin
CAS:520-36-5 |
|
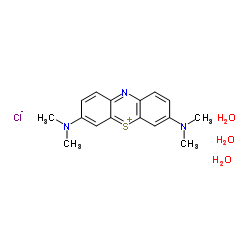 |
Methylene Blue trihydrate
CAS:7220-79-3 |
|
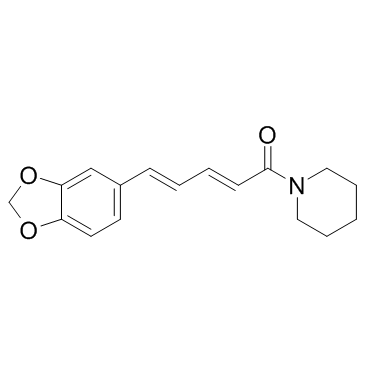 |
Piperine
CAS:94-62-2 |
|
 |
Harmane
CAS:486-84-0 |
|
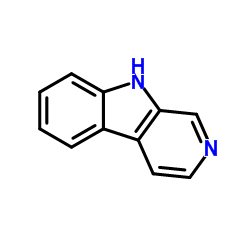 |
Norharmane
CAS:244-63-3 |