| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Ethanol
CAS:64-17-5 |
|
 |
Dimethyl sulfoxide
CAS:67-68-5 |
|
 |
Ibuprofen
CAS:15687-27-1 |
|
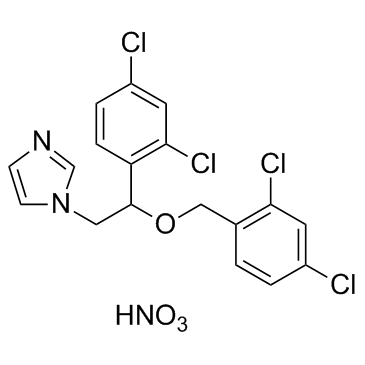 |
Miconazole Nitrate
CAS:22832-87-7 |
|
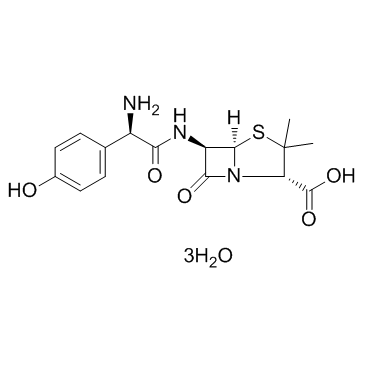 |
Amoxicillin Trihydrate
CAS:61336-70-7 |
|
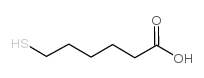 |
Hexanoic acid,6-mercapto
CAS:17689-17-7 |
|
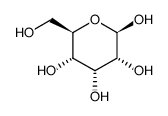 |
Beta-D-allose
CAS:7283-09-2 |
|
 |
8-Octanoyloxypyrene-1,3,6-trisulfonic acid trisodium salt
CAS:115787-84-3 |