| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Ethanol
CAS:64-17-5 |
|
 |
Glycerol
CAS:56-81-5 |
|
 |
sodium dodecyl sulfate
CAS:151-21-3 |
|
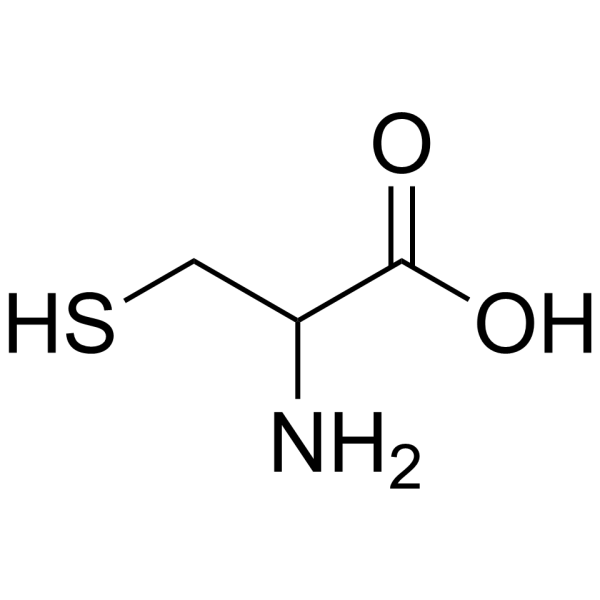 |
DL-CYSTEINE (1-13C)
CAS:3374-22-9 |
|
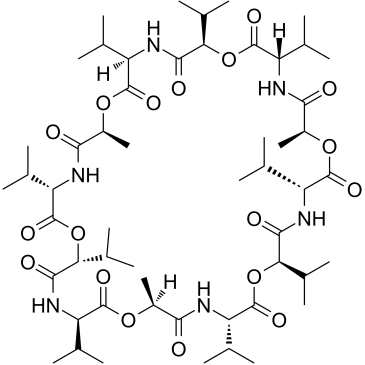 |
Valinomycin
CAS:2001-95-8 |
|
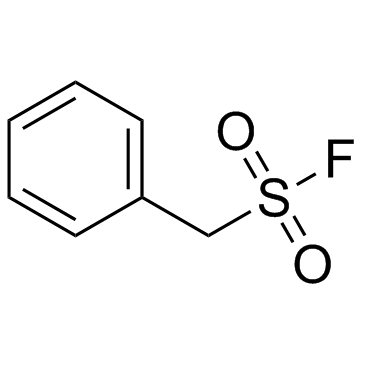 |
PMSF
CAS:329-98-6 |
|
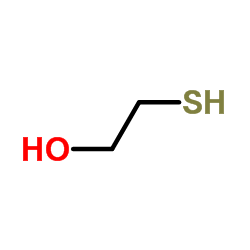 |
mercaptoethanol
CAS:60-24-2 |
|
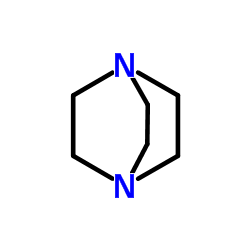 |
DABCO
CAS:280-57-9 |
|
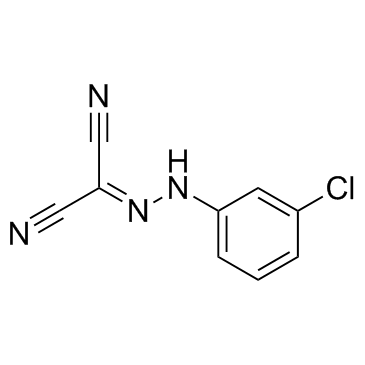 |
CCCP
CAS:555-60-2 |
|
 |
Chloramphenicol
CAS:56-75-7 |