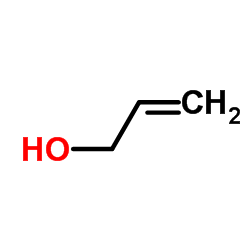
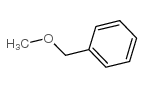
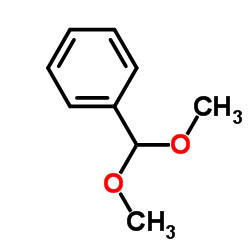
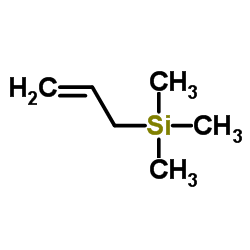
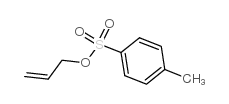
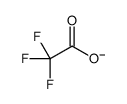
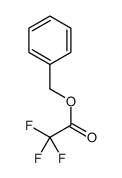
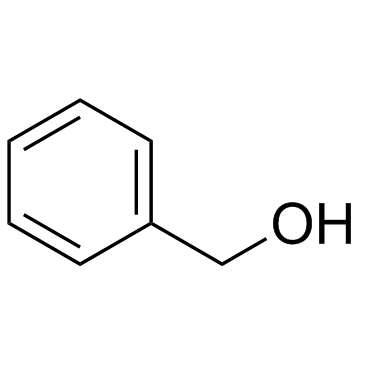
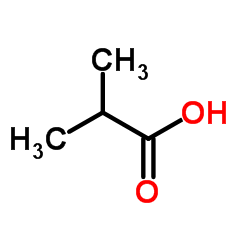
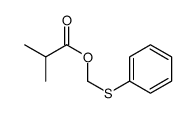
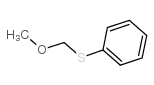
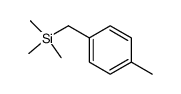
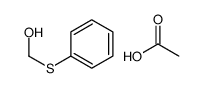
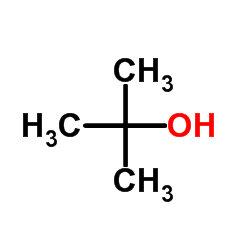
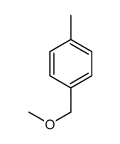
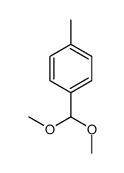
|
~35% |
|
~% |
|
~20% |
|
~24% |
|
~59% |
|
~67% |
|
~34% |
|
~67% |
|
~25% |
|
~8% |
|
~17% |
|
~% |
|
~% |
![o-[(trimethylsilyl)methyl]toluene Structure](https://image.chemsrc.com/caspic/147/4225-37-0.png)