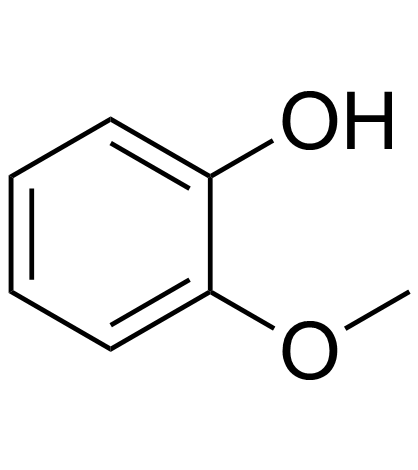
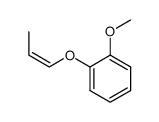
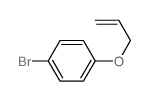
|
~58% |
|
~% |
|
~% |
|
~56% |
|
~% |
|
~79% |
|
~72% |
|
~78% |
|
~24% |
|
~% |
|
~69% |
|
~62% |
|
~85% |
|
~63% |
|
~% |
|
~46% |
|
~% |
|
~51% |
|
~46% |
|
~% |
|
~91% |
|
~68% |
|
~60% |
|
~69% |
|
~% |
|
~99% |
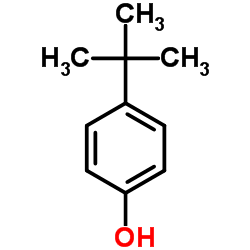
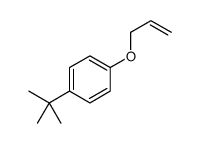
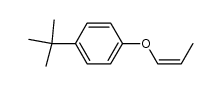



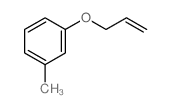

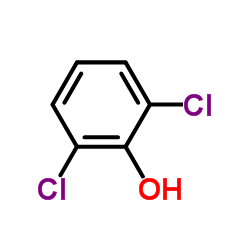
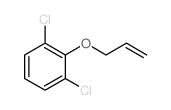
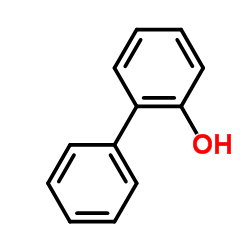
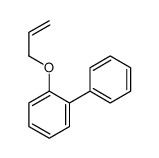
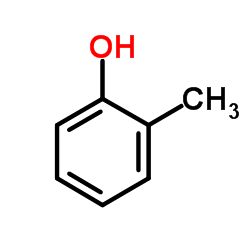
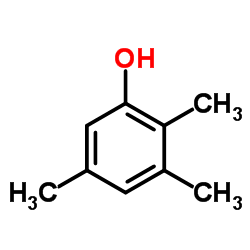

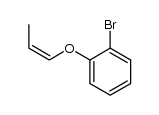
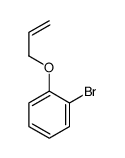


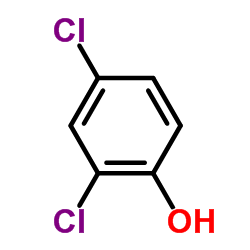
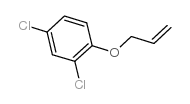

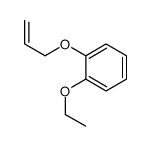
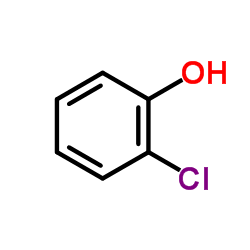
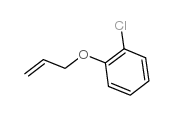
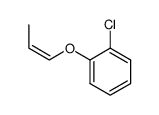


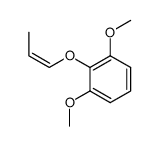
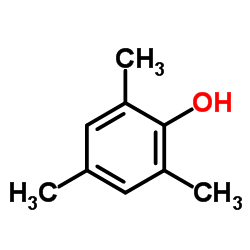
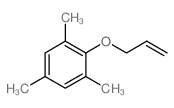
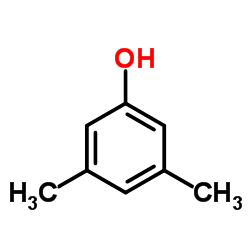

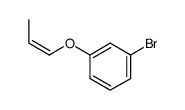
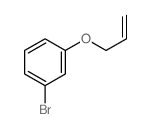
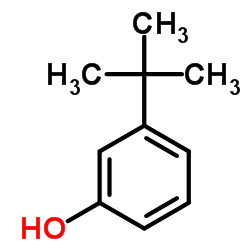

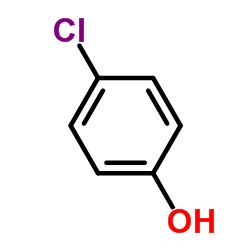
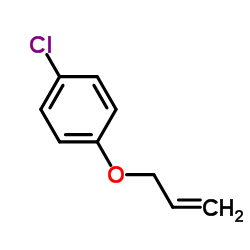


![Benzene,[(2-methyl-2-propen-1-yl)oxy] Structure](https://image.chemsrc.com/caspic/255/5820-22-4.png)