| Structure | Name/CAS No. | Articles |
|---|---|---|
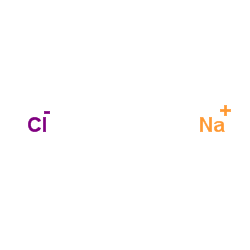 |
sodium chloride
CAS:7647-14-5 |
|
 |
L-γ-Glutamyl-S-nitroso-L-cysteinylglycine
CAS:57564-91-7 |
|
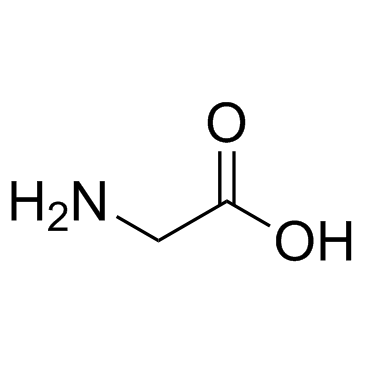 |
Glycine
CAS:56-40-6 |
|
 |
sodium dodecyl sulfate
CAS:151-21-3 |
|
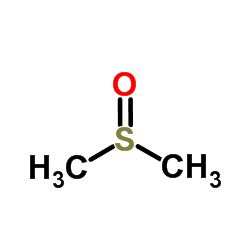 |
Dimethyl sulfoxide
CAS:67-68-5 |
|
 |
H-Arg(NO2)-OH
CAS:2149-70-4 |
|
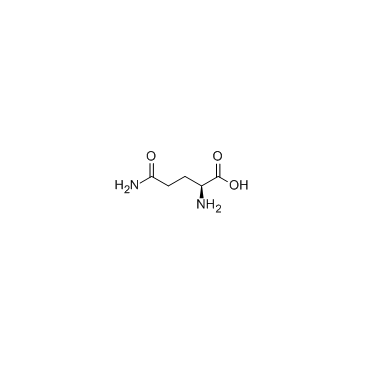 |
L-Glutamine
CAS:56-85-9 |
|
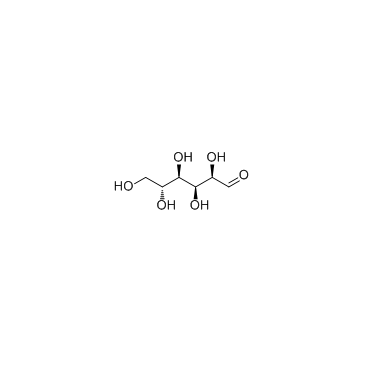 |
D-(+)-Glucose
CAS:50-99-7 |
|
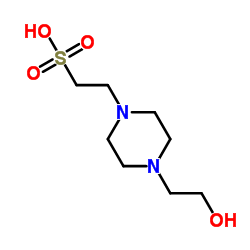 |
HEPES
CAS:7365-45-9 |
|
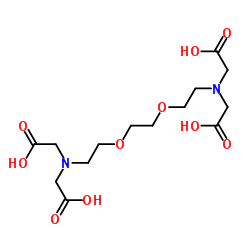 |
EGTA
CAS:67-42-5 |