| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
sucrose
CAS:57-50-1 |
|
 |
AEBSF HCl
CAS:30827-99-7 |
|
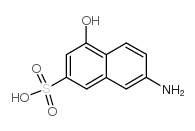 |
J acid
CAS:87-02-5 |
|
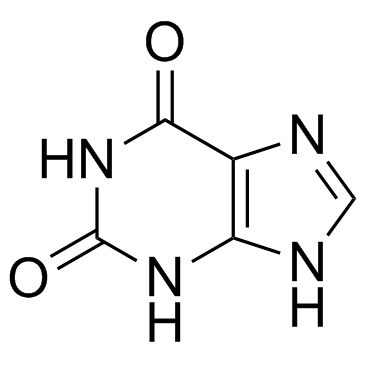 |
2,6-Dihydroxypurine
CAS:69-89-6 |
|
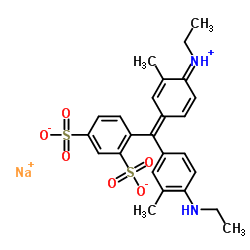 |
Xylene Cyanole F
CAS:2650-17-1 |
|
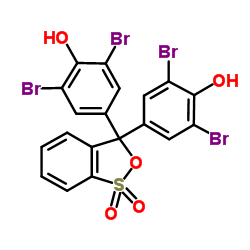 |
Bromophenol Blue
CAS:115-39-9 |
|
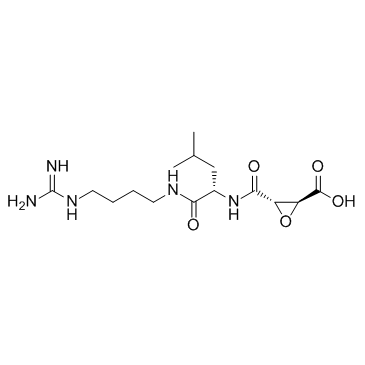 |
E-64
CAS:66701-25-5 |