| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Sodium hydroxide
CAS:1310-73-2 |
|
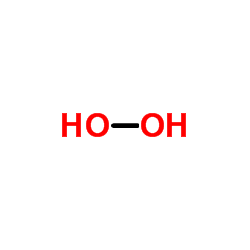 |
Hydrogen peroxide
CAS:7722-84-1 |
|
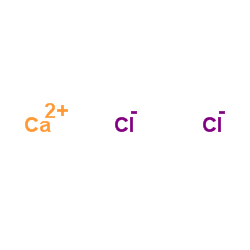 |
Calcium chloride
CAS:10043-52-4 |
|
 |
3-Ethyl-2,4-pentanedione
CAS:1540-34-7 |
|
 |
magnesium sulfate
CAS:7487-88-9 |
|
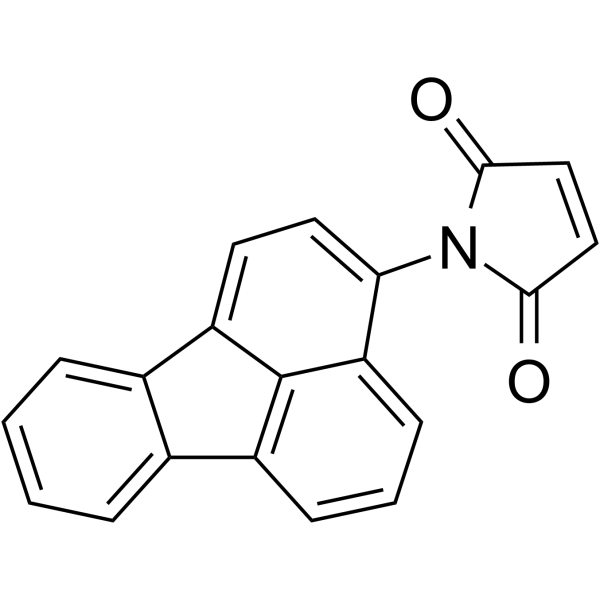 |
N-(3-Fluoranthyl)maleimide
CAS:60354-76-9 |
|
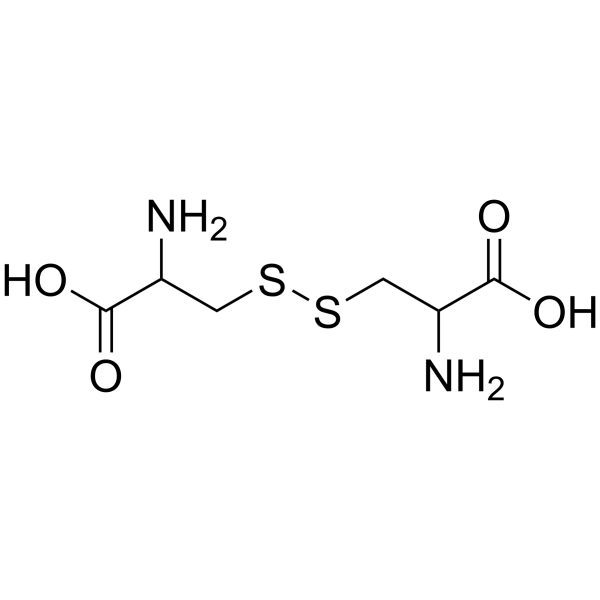 |
DL-Cystine
CAS:923-32-0 |
|
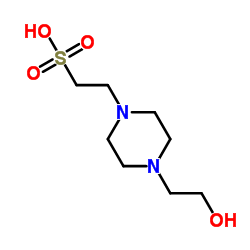 |
HEPES
CAS:7365-45-9 |
|
 |
gldh
CAS:9029-12-3 |
|
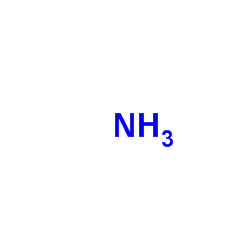 |
Ammonia
CAS:7664-41-7 |