| Structure | Name/CAS No. | Articles |
|---|---|---|
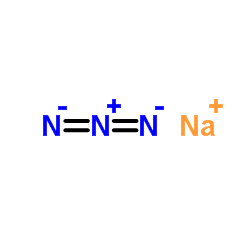 |
Sodium azide
CAS:26628-22-8 |
|
 |
DL-Lysine
CAS:70-54-2 |
|
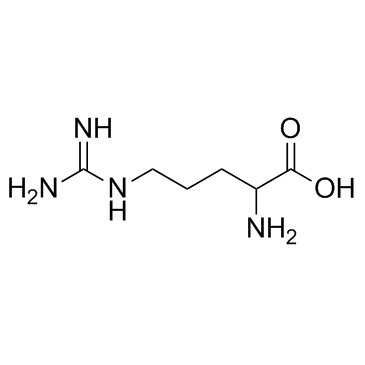 |
DL-Arginine
CAS:7200-25-1 |
|
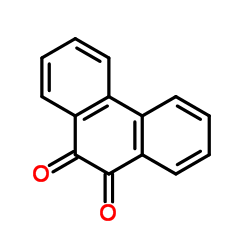 |
Phenanthrene-9,10-dione
CAS:84-11-7 |
|
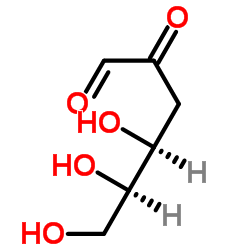 |
D-3-Deoxyglucosone
CAS:4084-27-9 |