| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Hydrochloric acid
CAS:7647-01-0 |
|
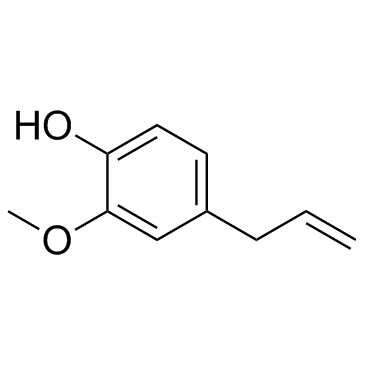 |
Eugenol
CAS:97-53-0 |
|
 |
Methanol
CAS:67-56-1 |
|
 |
Methyleugenol
CAS:93-15-2 |
|
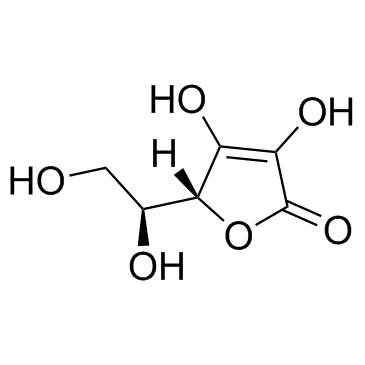 |
Ascorbic acid
CAS:50-81-7 |
|
 |
Dimethyl sulfoxide
CAS:67-68-5 |
|
 |
acetic acid
CAS:64-19-7 |
|
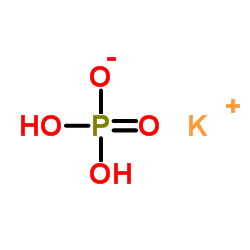 |
Monopotassium phosphate
CAS:7778-77-0 |
|
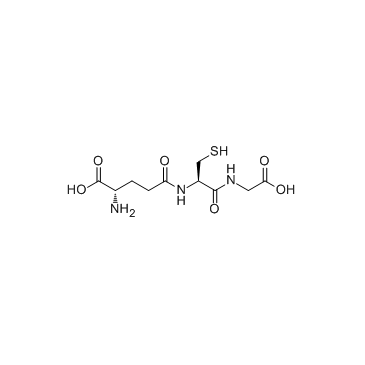 |
Glutathione
CAS:70-18-8 |
|
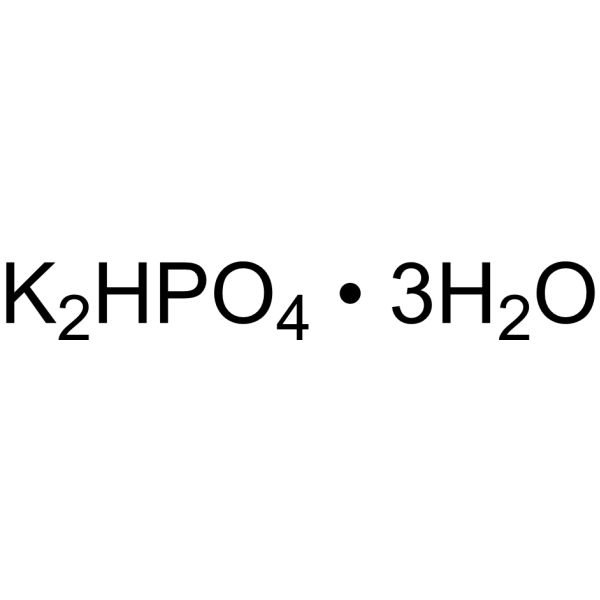 |
Potassium hydrogen phosphate hydrate (2:1:3)
CAS:16788-57-1 |