| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Chloroform
CAS:67-66-3 |
|
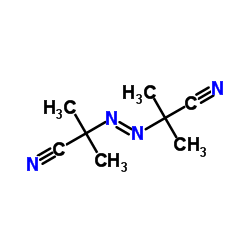 |
2,2'-Azobis(2-methylpropionitrile)
CAS:78-67-1 |
|
 |
Acetone
CAS:67-64-1 |
|
 |
N-hexane
CAS:110-54-3 |
|
 |
Acetonitrile
CAS:75-05-8 |
|
 |
Methanol
CAS:67-56-1 |
|
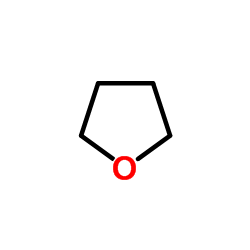 |
thf
CAS:109-99-9 |
|
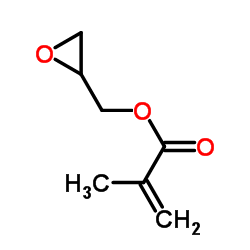 |
Glycidyl methacrylate
CAS:106-91-2 |
|
 |
Decan-1-ol
CAS:112-30-1 |
|
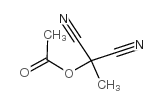 |
Propanedinitrile,2-(acetyloxy)-2-methyl
CAS:7790-01-4 |