| Structure | Name/CAS No. | Articles |
|---|---|---|
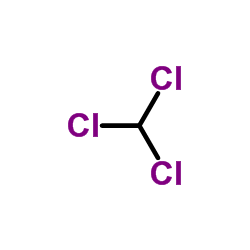 |
Chloroform
CAS:67-66-3 |
|
 |
N-hexane
CAS:110-54-3 |
|
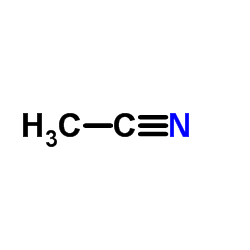 |
Acetonitrile
CAS:75-05-8 |
|
 |
Dichloromethane
CAS:75-09-2 |
|
 |
Ibuprofen
CAS:15687-27-1 |
|
 |
ethyl acetate
CAS:141-78-6 |
|
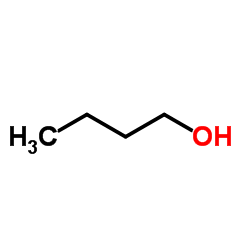 |
Butanol
CAS:71-36-3 |
|
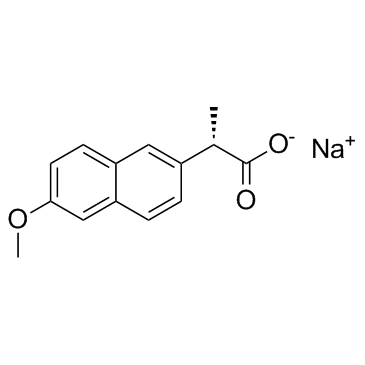 |
naproxen sodium
CAS:26159-34-2 |