| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Chloroform
CAS:67-66-3 |
|
 |
Methanol
CAS:67-56-1 |
|
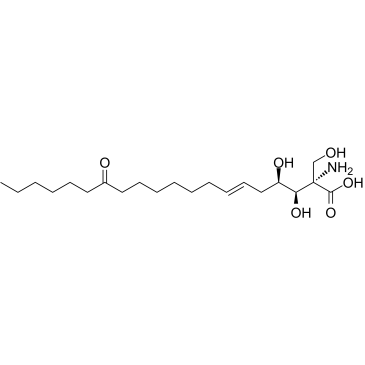 |
Myriocin
CAS:35891-70-4 |
|
 |
Glibenclamide
CAS:10238-21-8 |
|
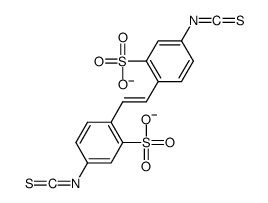 |
4,4'-Diisothiocyanatostilbene-2,2'-disulfonic acid disodium salt hydrate
CAS:207233-90-7 |
|
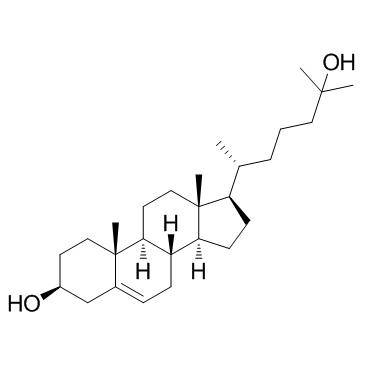 |
25-Hydroxycholesterol
CAS:2140-46-7 |