| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Chloroform
CAS:67-66-3 |
|
 |
N-hexane
CAS:110-54-3 |
|
 |
Methanol
CAS:67-56-1 |
|
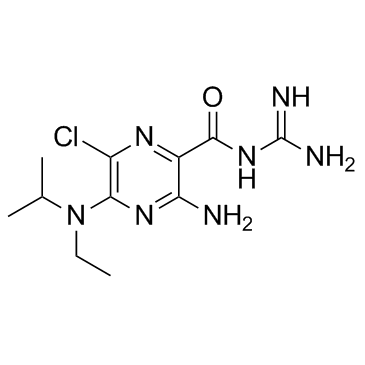 |
EIPA
CAS:1154-25-2 |
|
 |
ethyl acetate
CAS:141-78-6 |
|
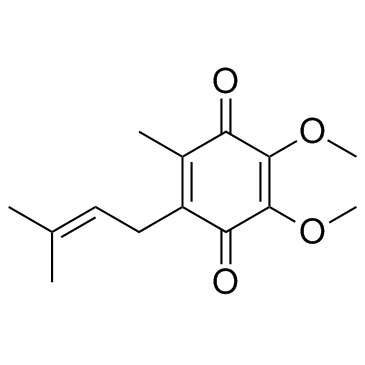 |
Ubiquinone-1
CAS:727-81-1 |
|
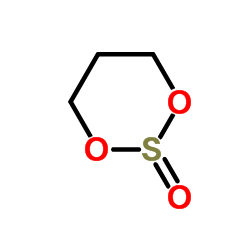 |
1,3,2-Dioxathiane 2-oxide
CAS:4176-55-0 |
|
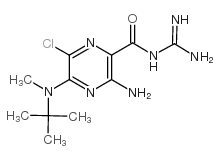 |
5-(N-Methyl-N-isobutyl)-Amiloride
CAS:96861-65-3 |