| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Chloroform
CAS:67-66-3 |
|
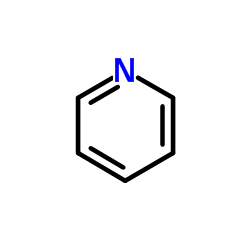 |
Pyridine
CAS:110-86-1 |
|
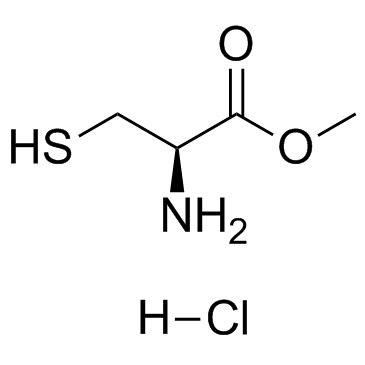 |
L-Cysteine methyl ester hydrochloride
CAS:18598-63-5 |
|
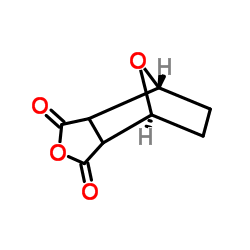 |
(±)-Norcantharidin
CAS:29745-04-8 |
|
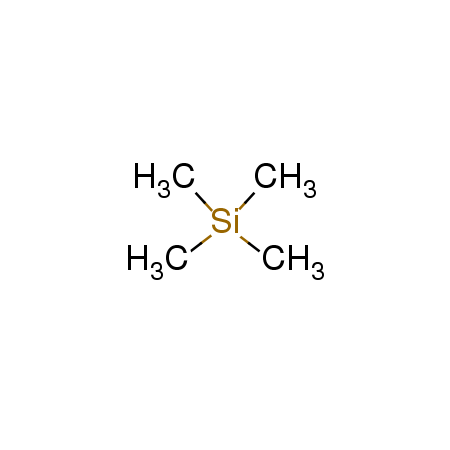 |
TMS
CAS:75-76-3 |
|
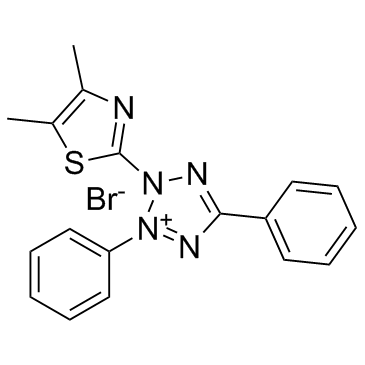 |
Thiazolyl Blue
CAS:298-93-1 |