| Structure | Name/CAS No. | Articles |
|---|---|---|
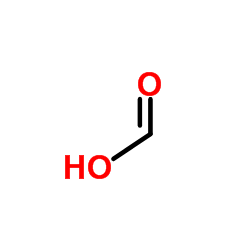 |
Formic Acid
CAS:64-18-6 |
|
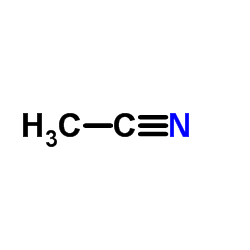 |
Acetonitrile
CAS:75-05-8 |
|
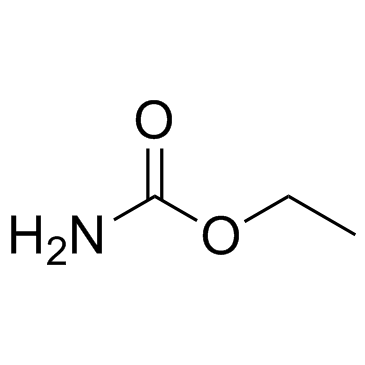 |
Urethane
CAS:51-79-6 |
|
 |
dimercaprol
CAS:59-52-9 |