| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Formic Acid
CAS:64-18-6 |
|
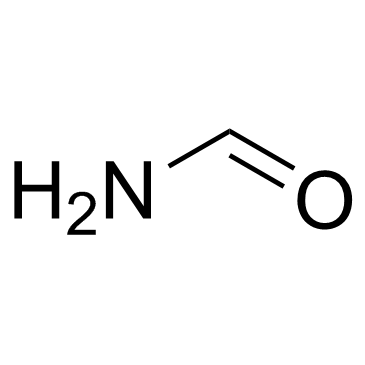 |
Formamide
CAS:75-12-7 |
|
 |
N,N-Dimethylformamide
CAS:68-12-2 |
|
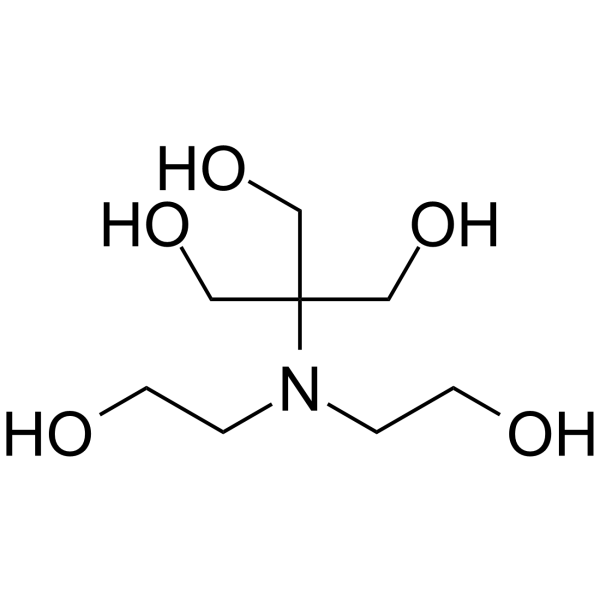 |
Bis-tris methane
CAS:6976-37-0 |
|
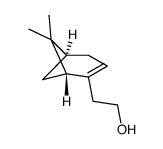 |
(-)-NOPOL
CAS:35836-73-8 |
|
 |
Hippuryl-Phe-OH
CAS:744-59-2 |