| Structure | Name/CAS No. | Articles |
|---|---|---|
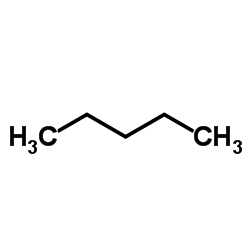 |
Pentane
CAS:109-66-0 |
|
 |
Diethyl ether
CAS:60-29-7 |
|
 |
N-hexane
CAS:110-54-3 |
|
 |
Dichloromethane
CAS:75-09-2 |
|
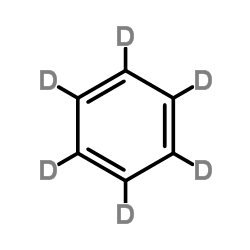 |
Benzene-d6
CAS:1076-43-3 |
|
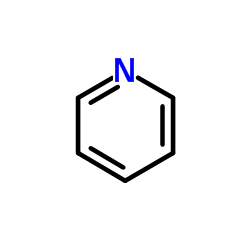 |
Pyridine
CAS:110-86-1 |
|
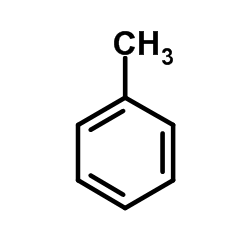 |
Toluene
CAS:108-88-3 |
|
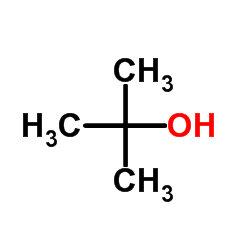 |
tert-Butanol
CAS:75-65-0 |
|
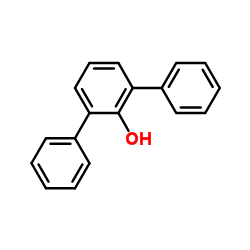 |
2,6-Diphenylphenol
CAS:2432-11-3 |