| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Sulfuric acid
CAS:7664-93-9 |
|
 |
Sodium hydroxide
CAS:1310-73-2 |
|
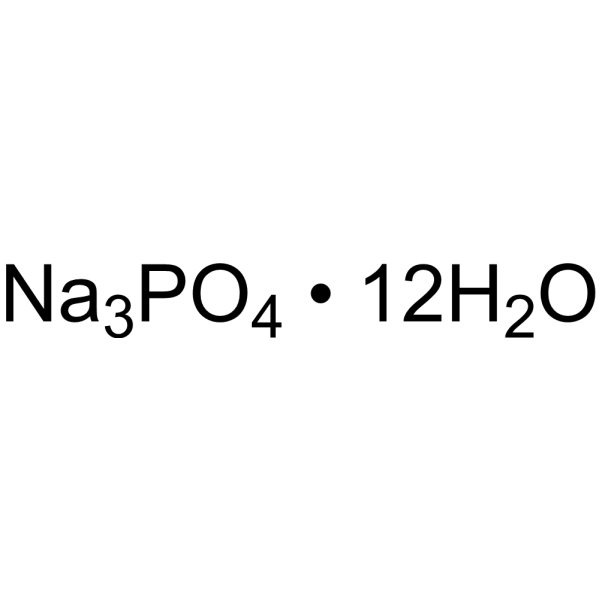 |
Trisodium phosphate dodecahydrate
CAS:10101-89-0 |
|
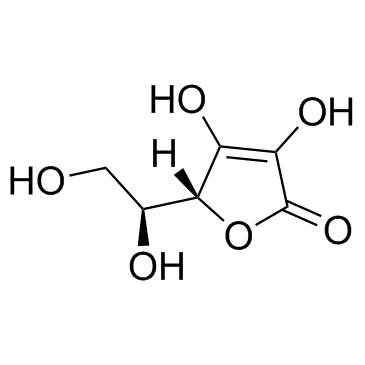 |
Ascorbic acid
CAS:50-81-7 |
|
 |
3-Ethyl-2,4-pentanedione
CAS:1540-34-7 |
|
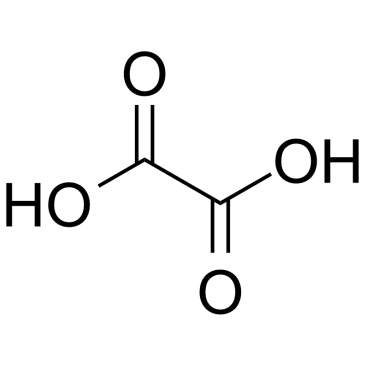 |
Oxalic acid
CAS:144-62-7 |
|
 |
Iron(II,III) oxide
CAS:1317-61-9 |
|
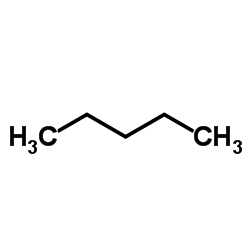 |
Pentane
CAS:109-66-0 |
|
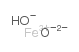 |
Iron hydroxide oxide
CAS:20344-49-4 |