| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
sodium carbonate
CAS:497-19-8 |
|
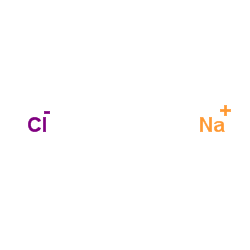 |
sodium chloride
CAS:7647-14-5 |
|
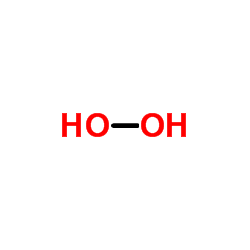 |
Hydrogen peroxide
CAS:7722-84-1 |
|
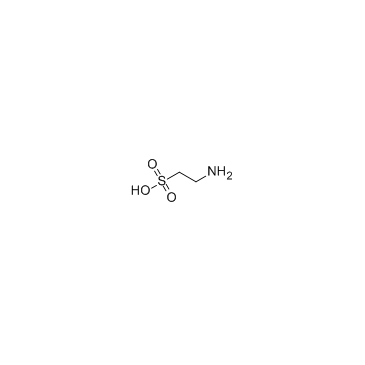 |
Taurine
CAS:107-35-7 |
|
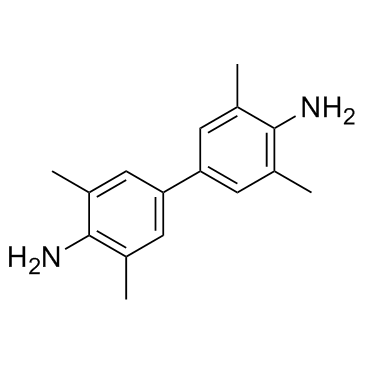 |
Tetramethylbenzidine
CAS:54827-17-7 |
|
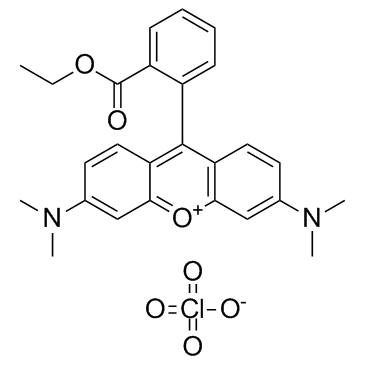 |
TMRE
CAS:115532-52-0 |
|
 |
Cyclosporin A
CAS:59865-13-3 |
|
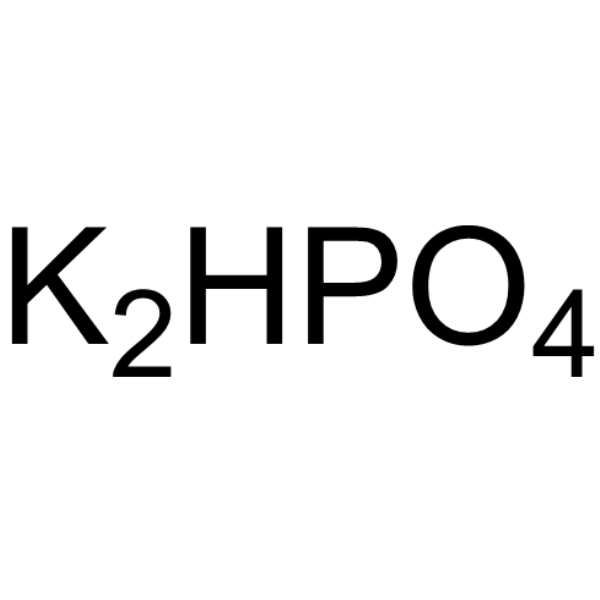 |
Di-potassium monohydrogen phosphate
CAS:7758-11-4 |
|
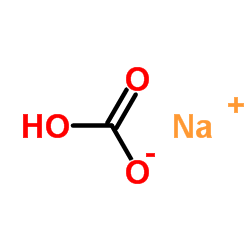 |
SodiuM bicarbonate
CAS:144-55-8 |
|
 |
Sodium selenite
CAS:10102-18-8 |