| Structure | Name/CAS No. | Articles |
|---|---|---|
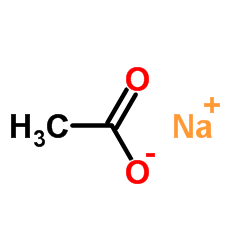 |
Sodium acetate
CAS:127-09-3 |
|
 |
Formic Acid
CAS:64-18-6 |
|
 |
Acetone
CAS:67-64-1 |
|
 |
Hydrochloric acid
CAS:7647-01-0 |
|
 |
sodium chloride
CAS:7647-14-5 |
|
 |
Acetonitrile
CAS:75-05-8 |
|
 |
Methanol
CAS:67-56-1 |
|
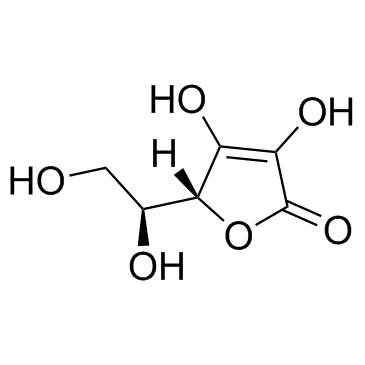 |
Ascorbic acid
CAS:50-81-7 |
|
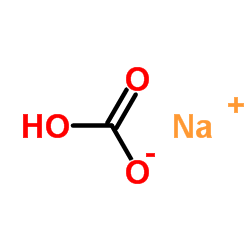 |
SodiuM bicarbonate
CAS:144-55-8 |
|
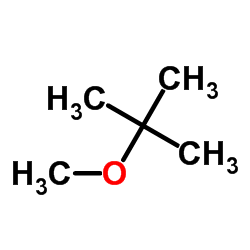 |
Methyl tert-butyl ether
CAS:1634-04-4 |