| Structure | Name/CAS No. | Articles |
|---|---|---|
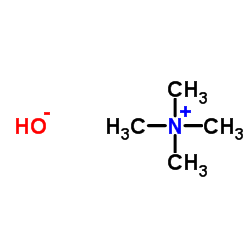 |
Tetramethylammonium hydroxide
CAS:75-59-2 |
|
 |
Ethanoic anhydride
CAS:108-24-7 |
|
 |
Tetrabutylammonium hydroxide
CAS:2052-49-5 |
|
 |
Dimethyl sulfoxide
CAS:67-68-5 |
|
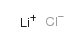 |
Lithium chloride
CAS:7447-41-8 |
|
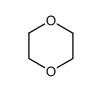 |
1,4-Dioxane
CAS:123-91-1 |
|
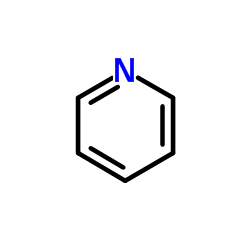 |
Pyridine
CAS:110-86-1 |
|
 |
N,N-Dimethylacetamide
CAS:127-19-5 |
|
 |
Sulfolane
CAS:126-33-0 |
|
 |
Acetyl chloride
CAS:75-36-5 |