| Structure | Name/CAS No. | Articles |
|---|---|---|
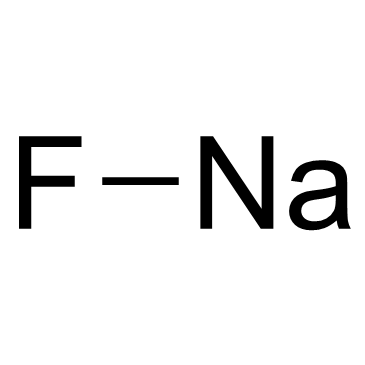 |
Sodium Fluoride
CAS:7681-49-4 |
|
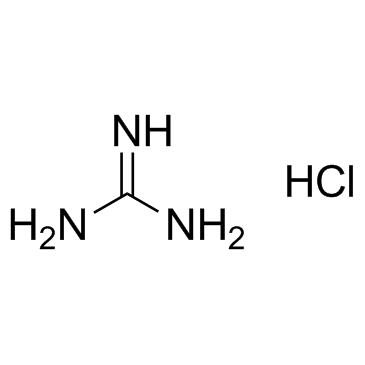 |
Guanidine hydrochloride
CAS:50-01-1 |
|
 |
sodium chloride
CAS:7647-14-5 |
|
 |
HEPES
CAS:7365-45-9 |
|
 |
SODIUM CHLORIDE-35 CL
CAS:20510-55-8 |
|
 |
Glycerol
CAS:56-81-5 |
|
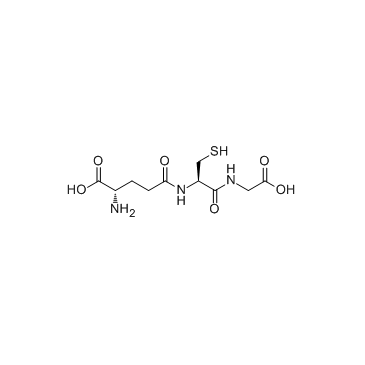 |
Glutathione
CAS:70-18-8 |
|
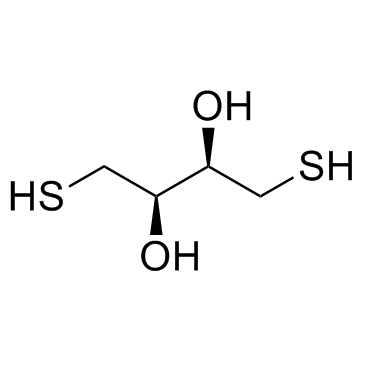 |
DL-Dithiothreitol
CAS:3483-12-3 |
|
 |
Ethylenediaminetetraacetic acid
CAS:60-00-4 |