| Structure | Name/CAS No. | Articles |
|---|---|---|
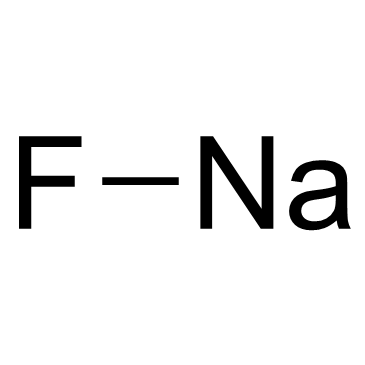 |
Sodium Fluoride
CAS:7681-49-4 |
|
 |
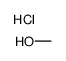
Hydrochloric acid
CAS:7647-01-0 |
|
 |
sodium chloride
CAS:7647-14-5 |
|
 |
Cyclosporin A
CAS:59865-13-3 |
|
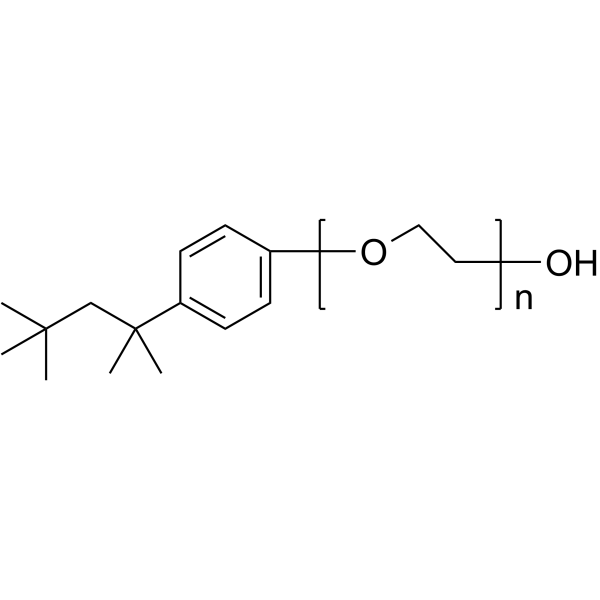 |
Triton X-100
CAS:9002-93-1 |
|
 |
SODIUM CHLORIDE-35 CL
CAS:20510-55-8 |
|
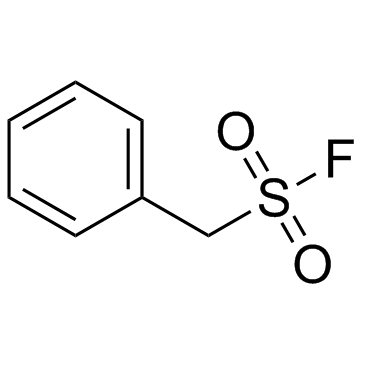 |
PMSF
CAS:329-98-6 |
|
 |
Ethylenediaminetetraacetic acid
CAS:60-00-4 |
|
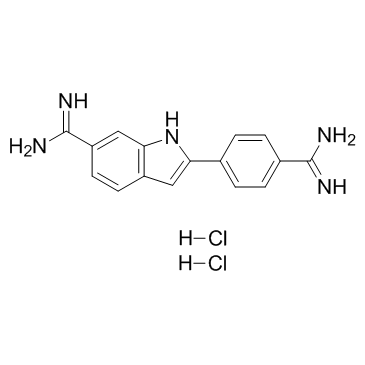 |
4',6-Diamidino-2-phenylindole dihydrochloride
CAS:28718-90-3 |
|
 |
HYDROGEN CHLORIDE ~1.25 M IN METHANOL, 250 ML
CAS:132228-87-6 |