| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Sulfuric acid
CAS:7664-93-9 |
|
 |
Ethanol
CAS:64-17-5 |
|
 |
titanium dioxide
CAS:13463-67-7 |
|
 |
Titanium oxide
CAS:1317-80-2 |
|
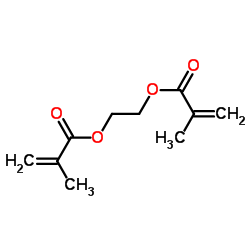 |
Ethylene methacrylate
CAS:97-90-5 |
|
 |
Titanium(IV) oxide, anatase
CAS:1317-70-0 |
|
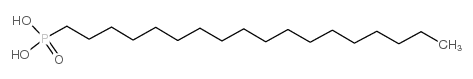 |
OCTADECYLPHOSPHONIC ACID
CAS:4724-47-4 |
|
 |
Glycerol
CAS:56-81-5 |
|
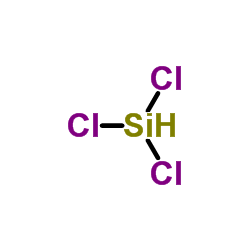 |
Trichlorosilane
CAS:10025-78-2 |
|
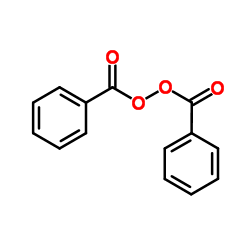 |
Benzoyl peroxide
CAS:94-36-0 |