| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Sulfuric acid
CAS:7664-93-9 |
|
 |
Ethanol
CAS:64-17-5 |
|
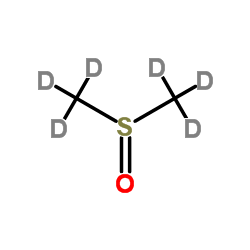 |
DIMETHYL SULFOXIDE-D6
CAS:2206-27-1 |
|
 |
Acetonitrile
CAS:75-05-8 |
|
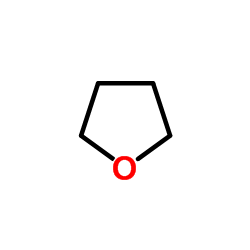 |
thf
CAS:109-99-9 |
|
 |
N,N-Dimethylformamide
CAS:68-12-2 |
|
 |
acetic acid
CAS:64-19-7 |
|
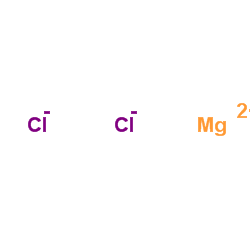 |
Magnesium choride
CAS:7786-30-3 |
|
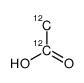 |
acetic acid
CAS:1173022-32-6 |
|
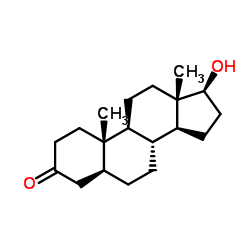 |
Stanolone
CAS:521-18-6 |