| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Sulfuric acid
CAS:7664-93-9 |
|
 |
Formic Acid
CAS:64-18-6 |
|
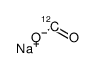 |
sodium,oxomethanolate
CAS:1218765-26-4 |
|
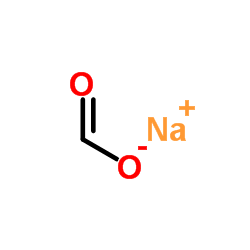 |
Sodium formate
CAS:141-53-7 |
|
 |
Sodium hydroxide
CAS:1310-73-2 |
|
 |
Ethanol
CAS:64-17-5 |
|
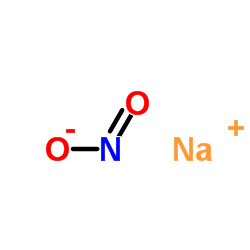 |
Sodium nitrite
CAS:7632-00-0 |
|
 |
Methanol
CAS:67-56-1 |
|
 |
Formaldehyde
CAS:50-00-0 |
|
 |
3-Ethyl-2,4-pentanedione
CAS:1540-34-7 |