| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Sulfuric acid
CAS:7664-93-9 |
|
 |
Ethanol
CAS:64-17-5 |
|
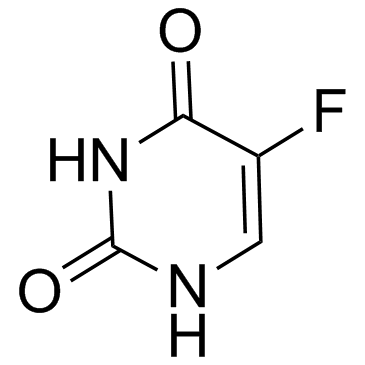 |
Fluorouracil
CAS:51-21-8 |
|
 |
Dimethyl sulfoxide
CAS:67-68-5 |
|
 |
Rapamycin (Sirolimus)
CAS:53123-88-9 |
|
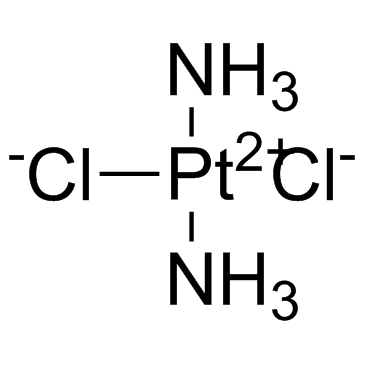 |
Cisplatin
CAS:15663-27-1 |
|
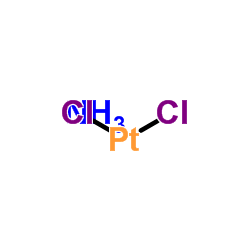 |
trans-Dichlorodiamineplatinum(II)
CAS:14913-33-8 |
|
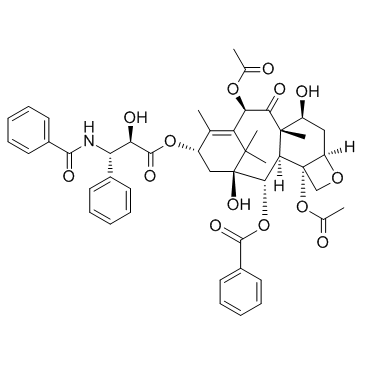 |
Paclitaxel
CAS:33069-62-4 |
|
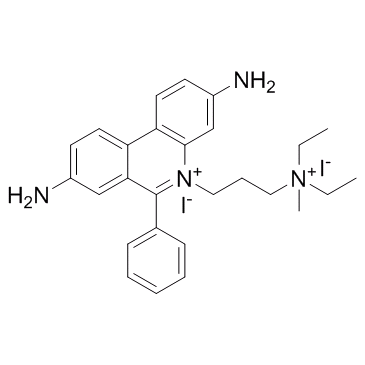 |
Propidium Iodide
CAS:25535-16-4 |
|
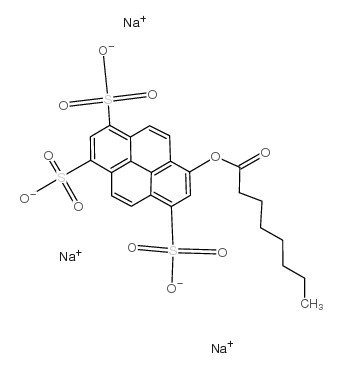 |
8-Octanoyloxypyrene-1,3,6-trisulfonic acid trisodium salt
CAS:115787-84-3 |