| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Glycerol
CAS:56-81-5 |
|
 |
Methanol
CAS:67-56-1 |
|
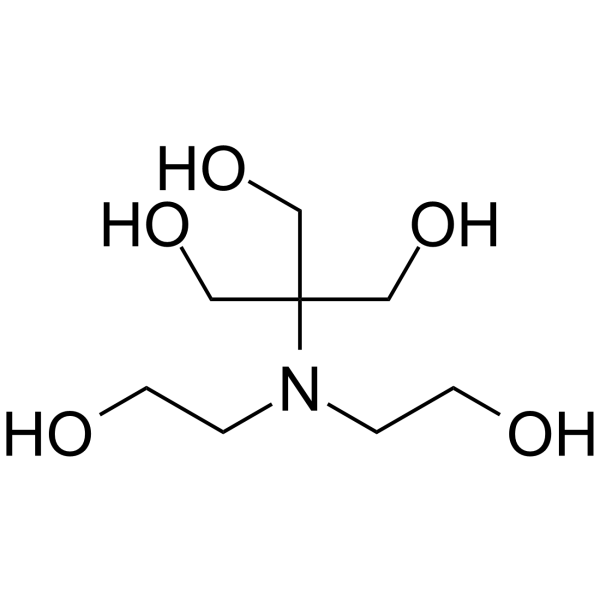 |
Bis-tris methane
CAS:6976-37-0 |
|
 |
HEPES
CAS:7365-45-9 |
|
 |
Ethylenediaminetetraacetic acid
CAS:60-00-4 |
|
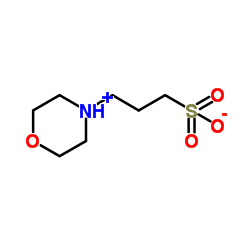 |
MOPS
CAS:1132-61-2 |
|
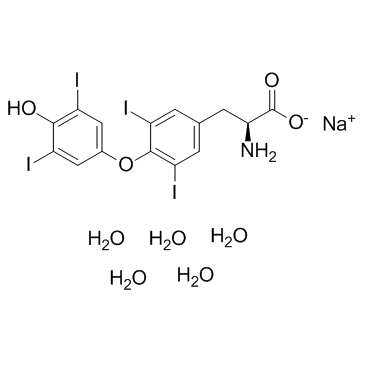 |
Sodium levothyroxine pentahydrate
CAS:6106-07-6 |
|
 |
trisodium phosphate
CAS:7601-54-9 |
|
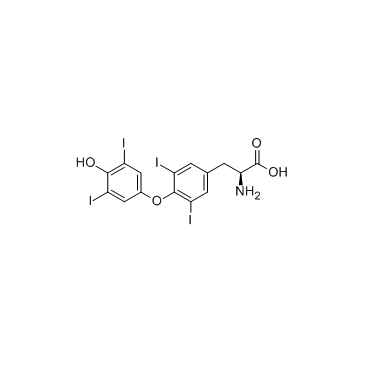 |
L-thyroxine
CAS:51-48-9 |