| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Glycerol
CAS:56-81-5 |
|
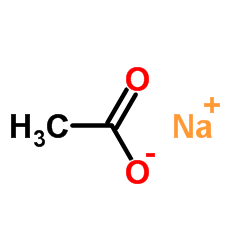 |
Sodium acetate
CAS:127-09-3 |
|
 |
HEPES
CAS:7365-45-9 |
|
 |
7-Aminoactinomycin D
CAS:7240-37-1 |