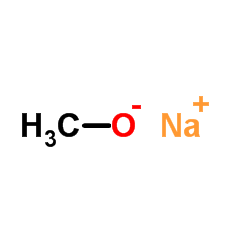
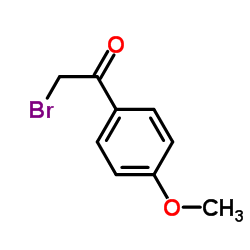
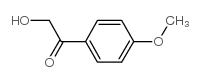
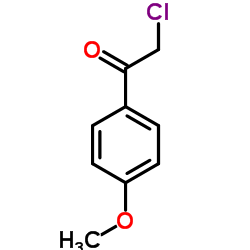
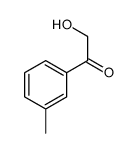
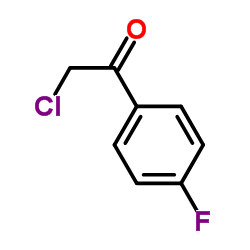
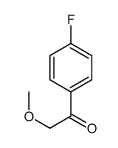
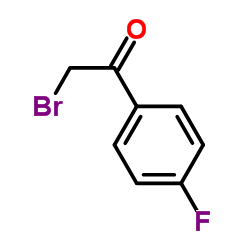
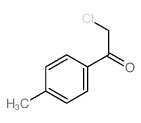
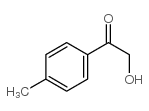
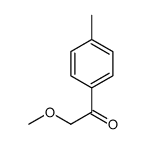
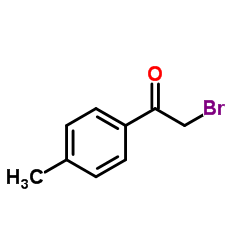
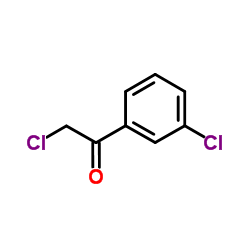
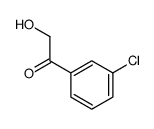
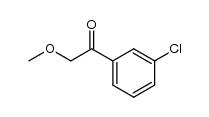
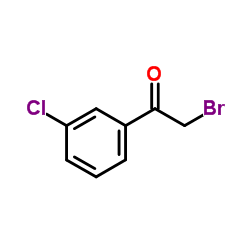
|
~30% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~71% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~88% |
|
~93% |
|
~% |
|
~% |
|
~% |
|
~14% |
|
~% |
|
~% |
|
~80% |
|
~% |
|
~% |
|
~% |