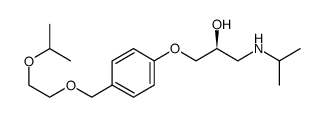
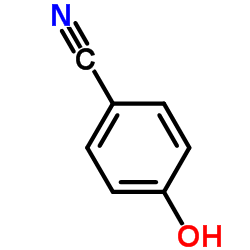
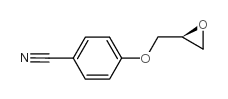
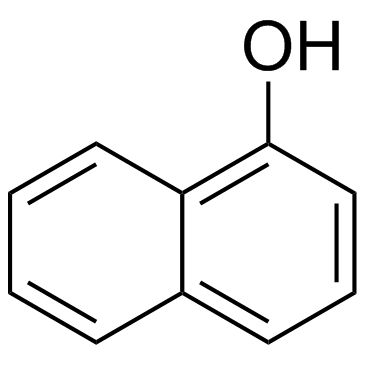
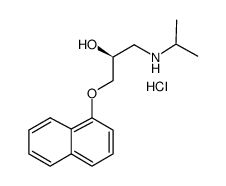
|
~% |
|
~89% |
|
~90% |
|
~88% |
|
~10% |
|
~85% |

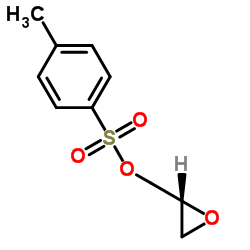
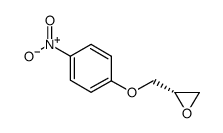

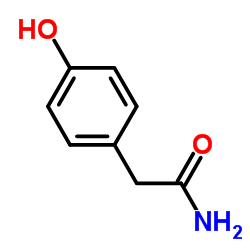
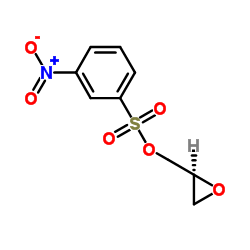
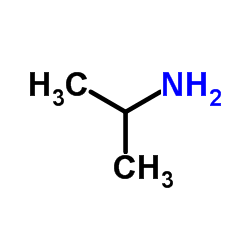
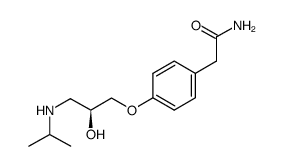
![4-[(2-Isopropoxyethoxy)methyl]phenol Structure](https://image.chemsrc.com/caspic/234/177034-57-0.png)