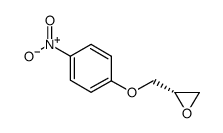
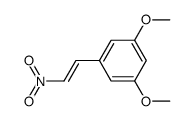
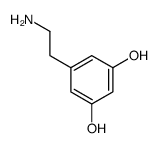
|
~82% |
|
~90% |
|
~92% |
|
~79% |
|
~93% |
|
~% |
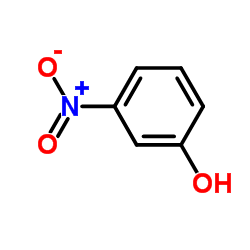
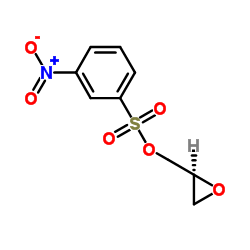

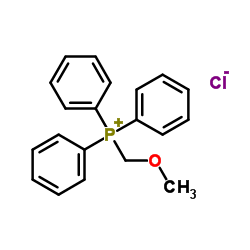
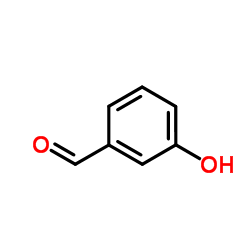
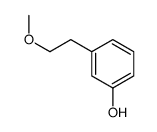
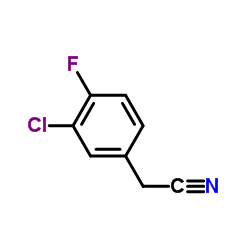

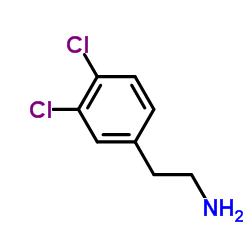
![(S)-2-[(4-methylsulfonylaminophenoxy)methyl]oxirane Structure](https://image.chemsrc.com/caspic/311/392620-41-6.png)