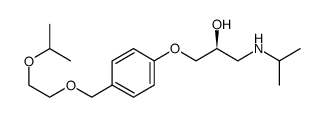
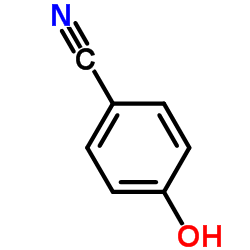
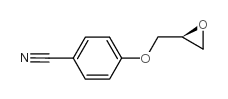
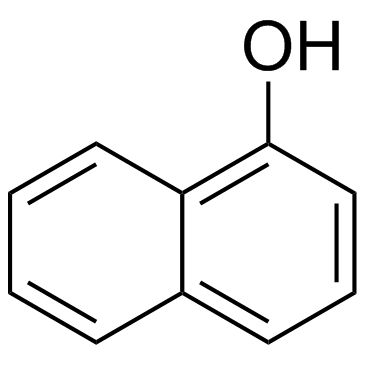
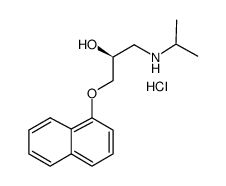
|
~% |
|
~89% |
|
~90% |
|
~88% |
|
~10% |
|
~85% |

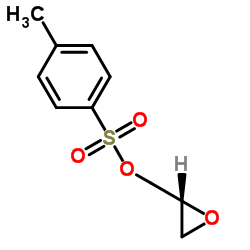
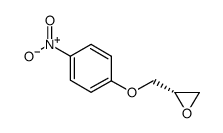

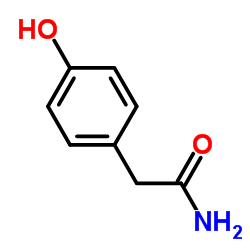
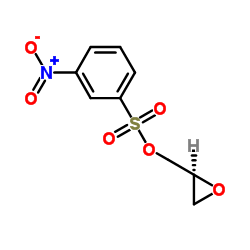
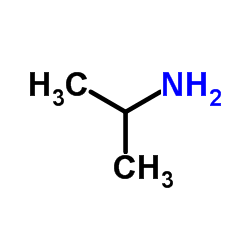
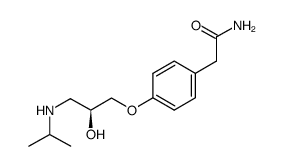
![4-[2-(1-甲基乙氧基)乙氧基]甲基苯酚 (比索洛尔中间体)结构式](https://image.chemsrc.com/caspic/234/177034-57-0.png)