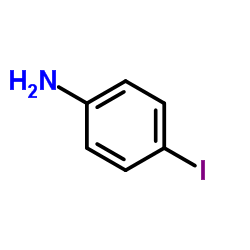
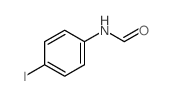
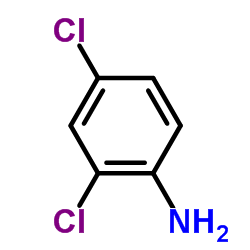
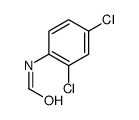
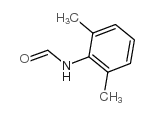
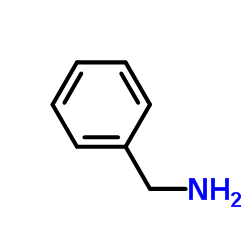
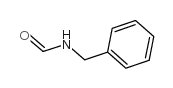
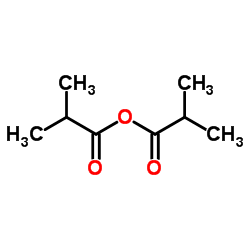
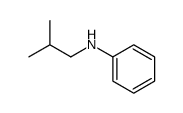
|
~99% |
|
~99% |
|
~97% |
|
~99% |
|
~94% |
|
~% |
|
~99% |
|
~10% |
|
~96% |
|
~99% |
|
~97% |
|
~99% |
|
~98% |
|
~% |
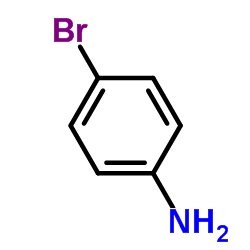
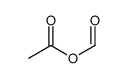
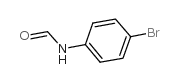
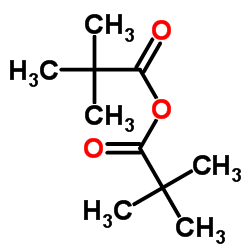

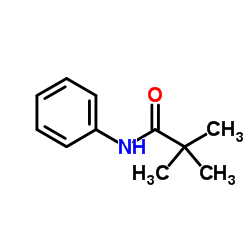
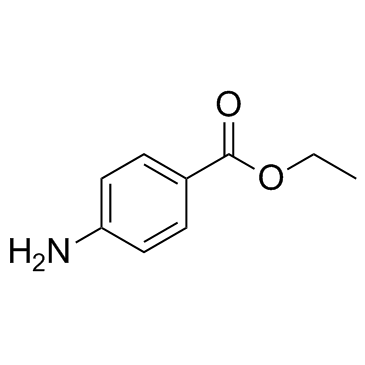
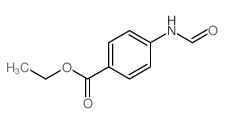
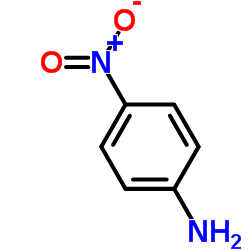
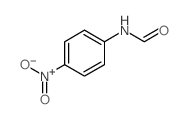
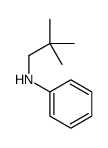
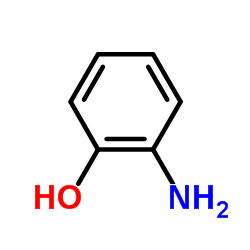
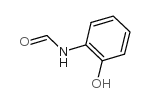
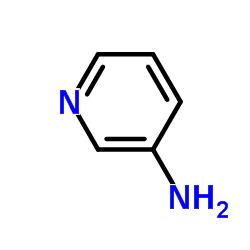
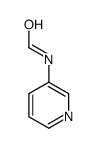
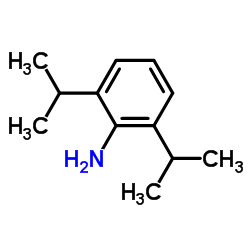
![N-[2,6-di(propan-2-yl)phenyl]formamide Structure](https://image.chemsrc.com/caspic/043/84250-69-1.png)