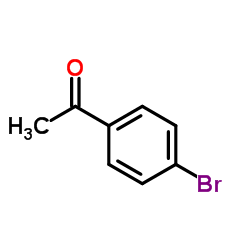
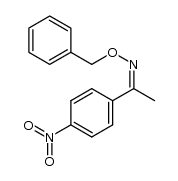
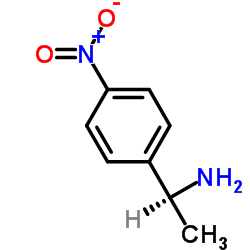
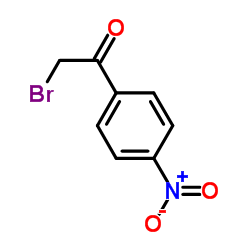
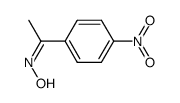
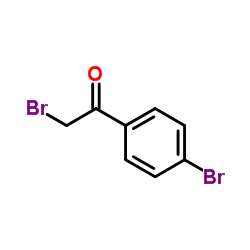
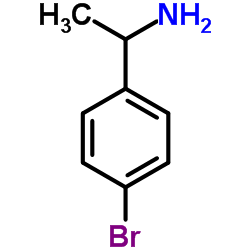
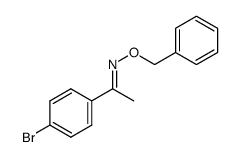
|
~77% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~68% |
|
~% |
|
~% |
|
~76% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~76% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |


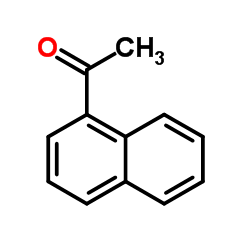

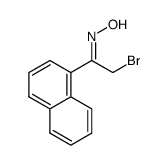

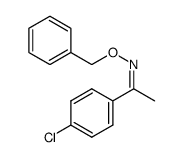
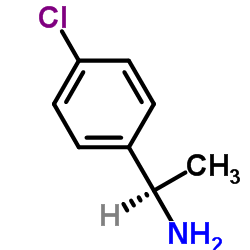


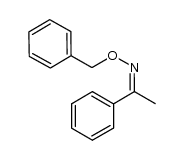
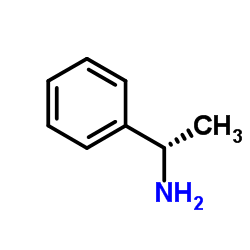
![Acetophenone [(Z)-oxime] Structure](https://image.chemsrc.com/caspic/239/50314-86-8.png)

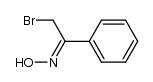

![N-[(1R)-1-(4-bromophenyl)ethyl]acetamide Structure](https://image.chemsrc.com/caspic/112/177750-53-7.png)