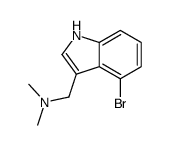
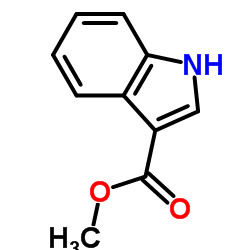
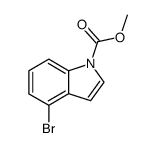
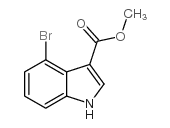
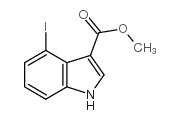
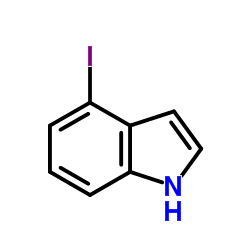
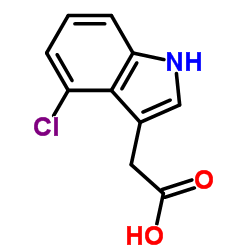
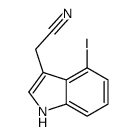
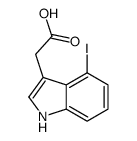
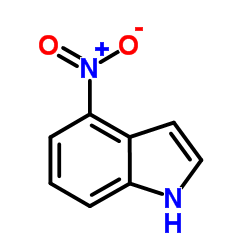
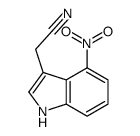
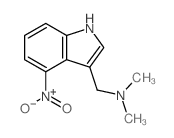
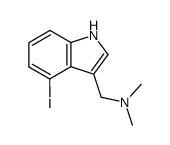
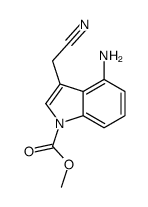
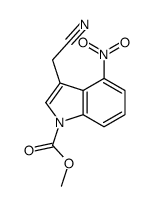
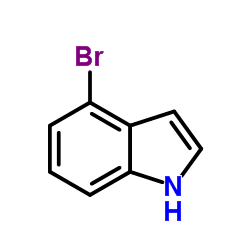
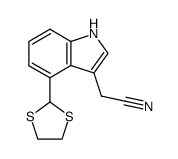
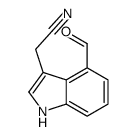
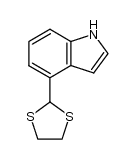
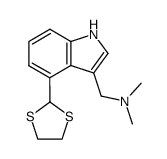
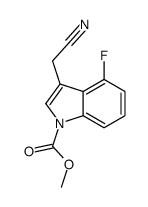
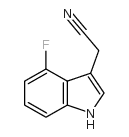
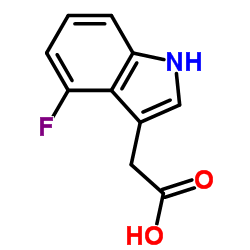
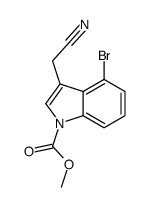
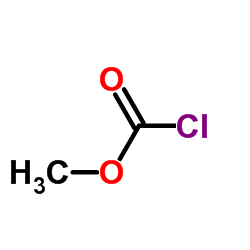
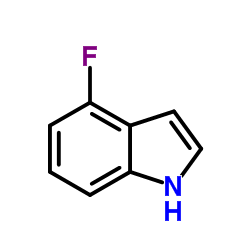
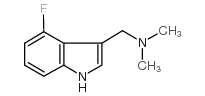
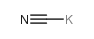|
~89% |
|
~% |
|
~% |
|
~% |
|
~90% |
|
~79% |
|
~96% |
|
~66% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~56% |
|
~67% |
|
~83% |
|
~94% |
|
~% |
|
~% |
|
~% |
|
~67% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~85% |
|
~55% |
|
~94% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~75% |
|
~% |
|
~% |
|
~% |
|
~89% |
|
~% |
|
~% |
|
~% |
|
~83% |
|
~66% |
|
~87% |
|
~% |