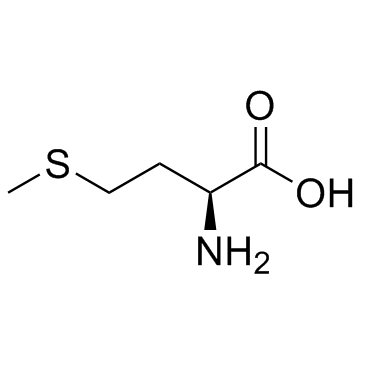
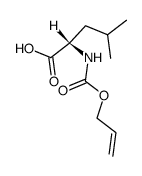
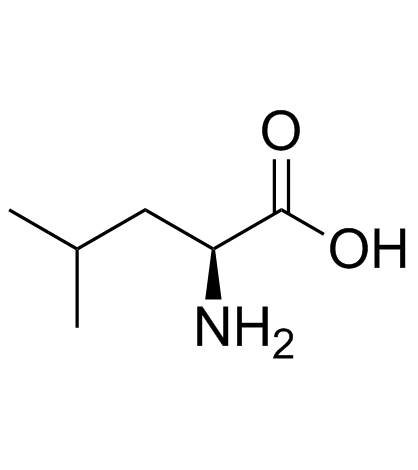
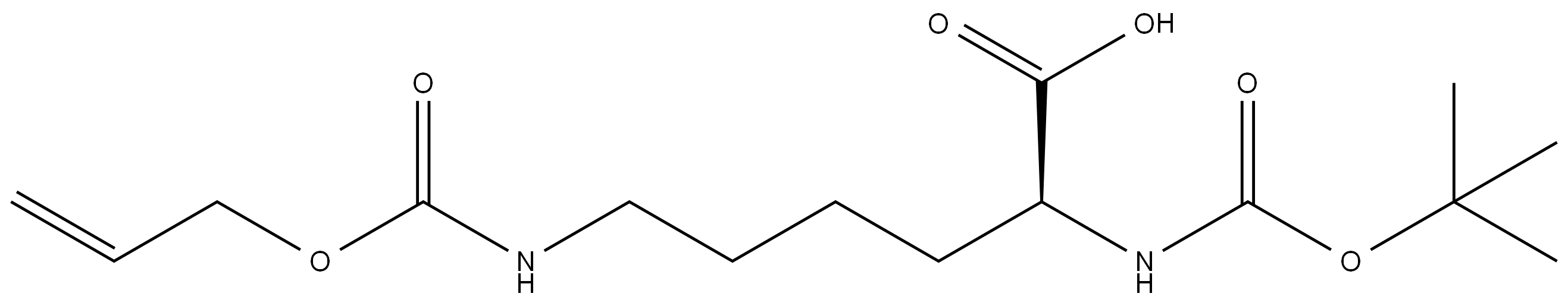
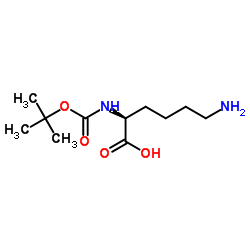
|
~82% |
|
~95% |
|
~% |
|
~94% |
|
~94% |
|
~95% |
|
~97% |
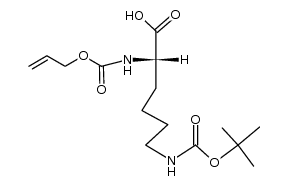
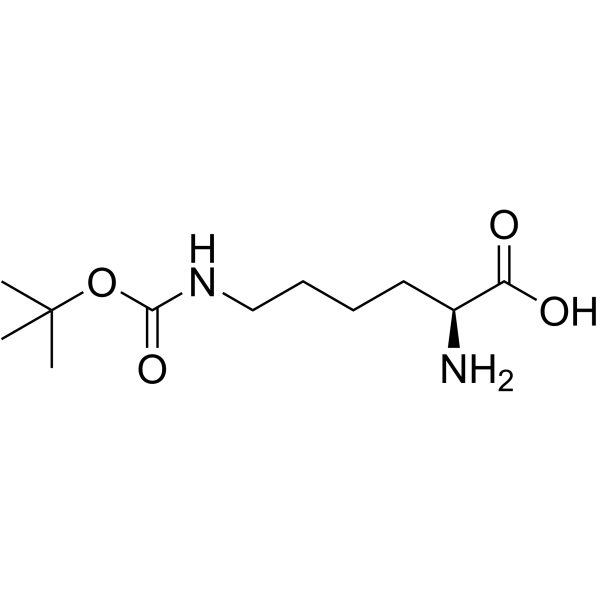
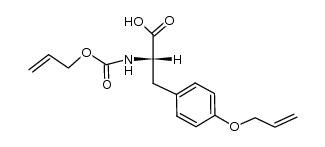
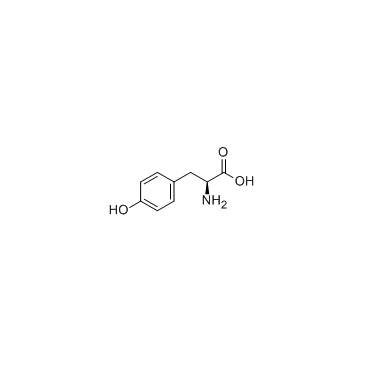
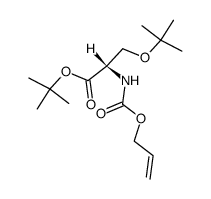
![tert-butyl 2-amino-3-[(2-methylpropan-2-yl)oxy]propanoate Structure](https://image.chemsrc.com/caspic/410/48067-24-9.png)
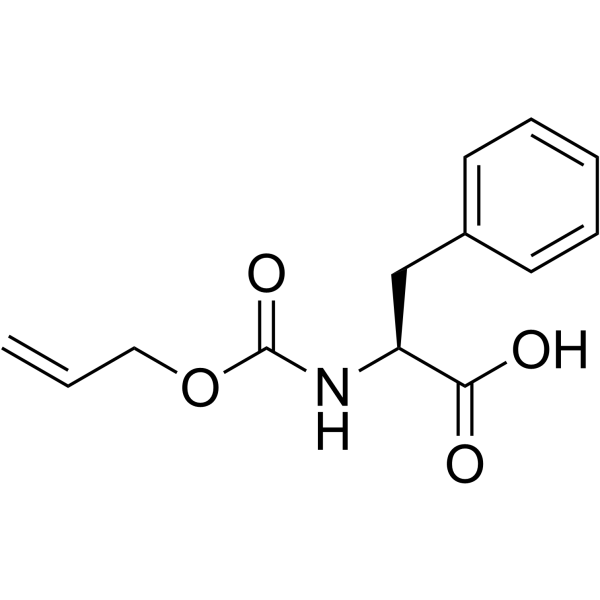

![L-Methionine, N-[(2-propen-1-yloxy)carbonyl] Structure](https://image.chemsrc.com/caspic/260/90508-21-7.png)