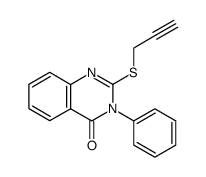
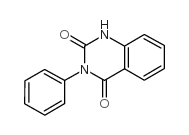
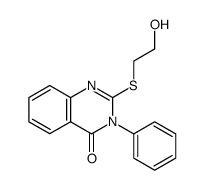
|
~24% |
|
~20% |
|
~46% |
|
~29% |
|
~14% |

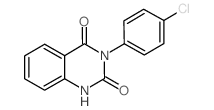

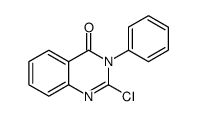
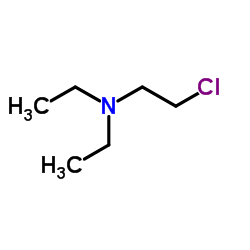
![2-[[2-(Diethylamino)ethyl]thio]-3-phenylquinazolin-4(3H)-one Structure](https://image.chemsrc.com/caspic/266/18619-72-2.png)