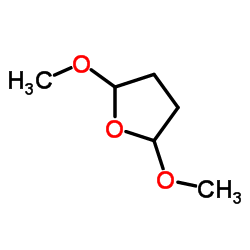
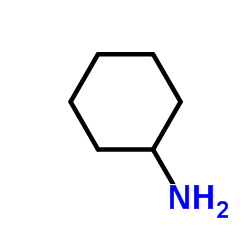
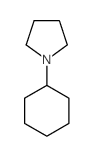
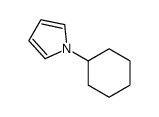
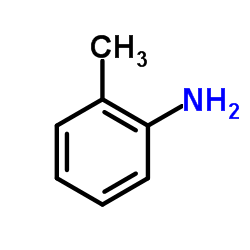
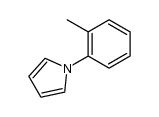
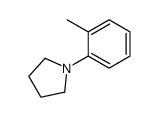
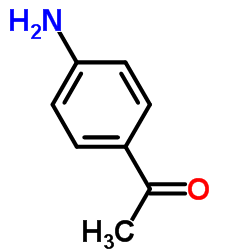
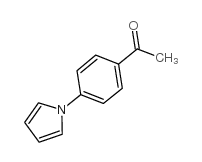
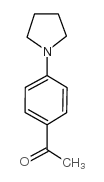
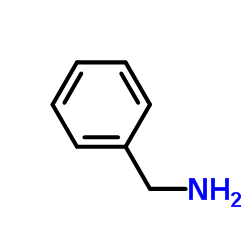
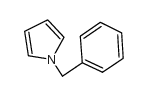
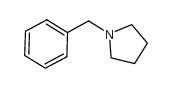
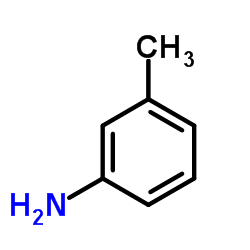
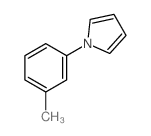
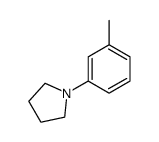
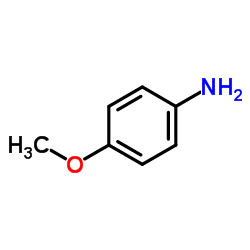
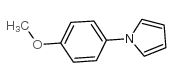
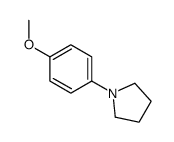
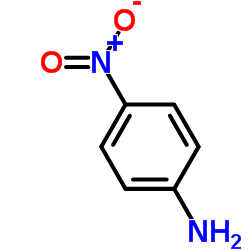
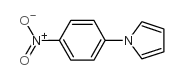
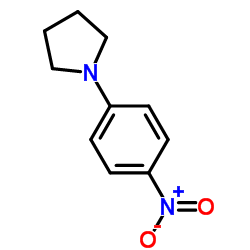
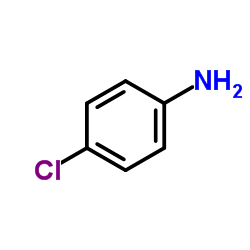
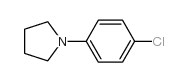
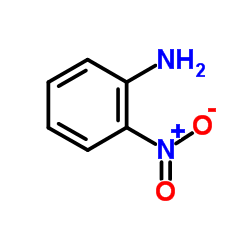
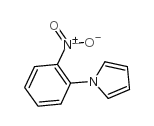
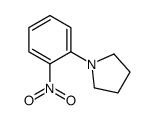
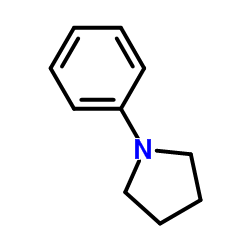
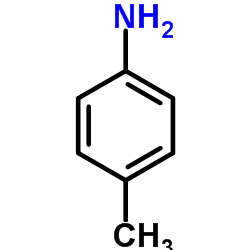
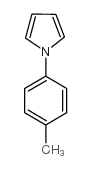
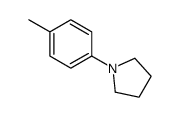
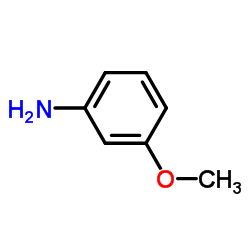
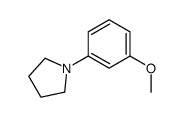
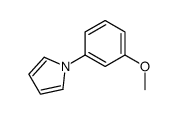
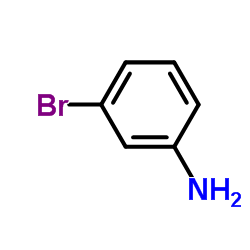
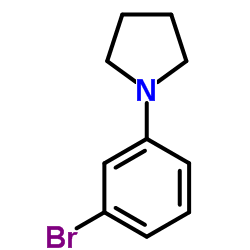
|
~36% |
|
~81% |
|
~0% |
|
~38% |
|
~0% |
|
~0% |
|
~0% |
|
~88% |
|
~83% |
|
~0% |
|
~87% |
|
~87% |
|
~0% |
|
~0% |
|
~78% |
|
~84% |
|
~0% |
|
~0% |
|
~65% |
|
~0% |
|
~0% |