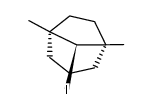
|
~%
Detail
|
|
~% |
|
~% |
|
~% |

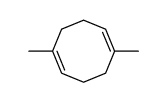
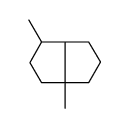
![anti-8-formoxy-1,5-dimethylbicyclo[3.2.1]octane Structure](https://image.chemsrc.com/caspic/397/74457-05-9.png)
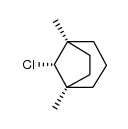
![syn-8-chloro-1,5-dimethylbicyclo[3.2.1]octane Structure](https://image.chemsrc.com/caspic/368/66729-68-8.png)
![anti-8-bromo-1,5-dimethylbicyclo[3.2.1]octane Structure](https://image.chemsrc.com/caspic/211/75911-75-0.png)
![anti-8-iodo-1,5-dimethylbicyclo[3.2.1]octane Structure](https://image.chemsrc.com/caspic/173/75911-76-1.png)