First-principles calculation of activity and selectivity of the partial oxidation of ethylene glycol on Fe(0 0 1), Co(0 0 0 1), and Ni(1 1 1)
Nobuki Ozawa, Shigeki Chieda, Yuji Higuchi, Tatsuya Takeguchi, Miho Yamauchi, Momoji Kubo
Index: 10.1016/j.jcat.2018.03.017
Full Text: HTML
Abstract
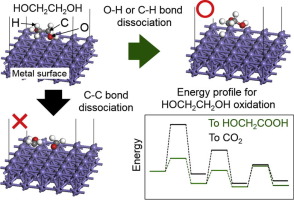
To recycle ethylene glycol (HOCH2CH2OH) fuel in alkaline fuel cells, active and selective catalysts for partially oxidizing HOCH2CH2OH to glycolic acid (HOCH2COOH) and oxalic acid ((COOH)2) are required at the anode; in other words, complete oxidation of HOCH2CH2OH to CO2 prevents ethylene glycol recycling. We investigate catalyst activity and selectivity for oxidizing HOCH2CH2OH to HOCH2COOH on Fe(0 0 1), Co(0 001), and Ni(1 1 1) via first-principles calculations. We calculate the oxidation reaction path from HOCH2CH2OH to HOCH2COOH without C 


















|
Combined quantitative FTIR and online GC study of Fischer-Tr...
2018-04-06 [10.1016/j.jcat.2018.03.026] |
|
Ethylene versus ethane: A DFT-based selectivity descriptor f...
2018-04-06 [10.1016/j.jcat.2018.03.019] |
|
Mechanistic insight into cobalt-catalyzed stereodivergent se...
2018-04-06 [10.1016/j.jcat.2018.03.016] |
|
Principles determining the activity of magnetic oxides for e...
2018-03-30 [10.1016/j.jcat.2018.03.012] |
|
Synthesis and characterization of Ag@Carbon core-shell spher...
2018-03-30 [10.1016/j.jcat.2018.02.029] |