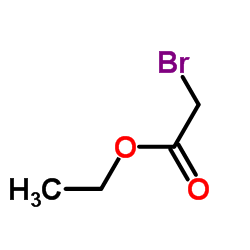
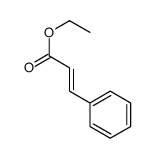
|
~69% |
|
~92% |
|
~70% |
|
~51% |
|
~41% |
|
~% |
![[(Trimethylsilyl)Methyl]Magnesium Chloride Structure](https://image.chemsrc.com/caspic/432/13170-43-9.png)

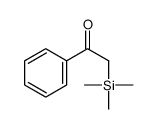
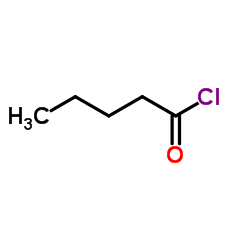



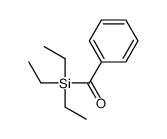
![[dimethyl(phenyl)silyl]-phenylmethanone Structure](https://image.chemsrc.com/caspic/214/17909-51-2.png)