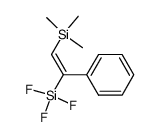
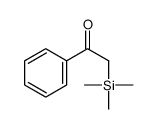
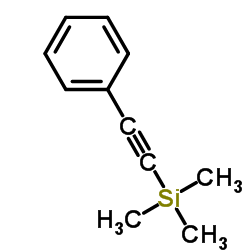
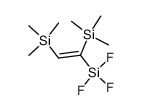
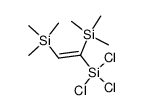
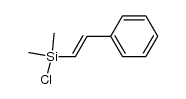
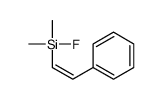
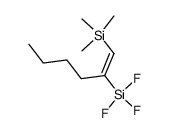
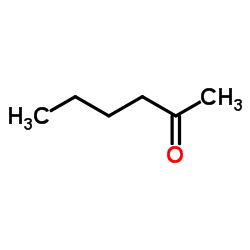
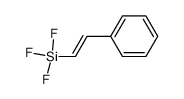
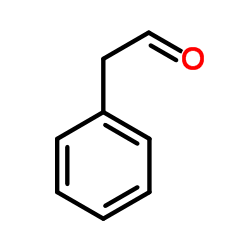
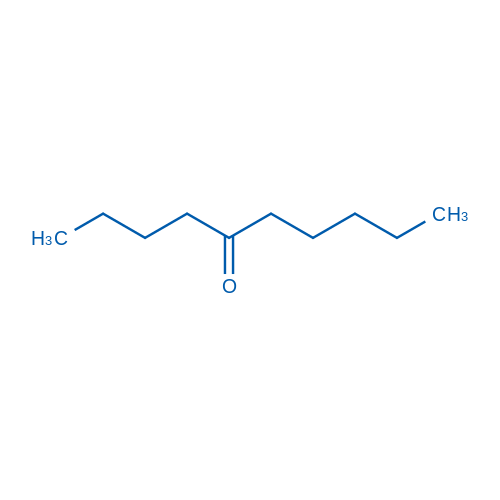
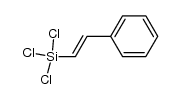
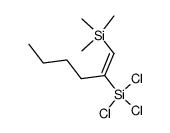
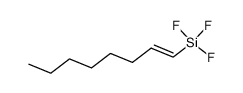
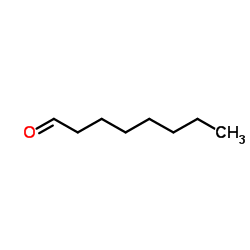
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~33% |
|
~0% |
|
~73% |
|
~76% |
|
~% |
|
~% |
|
~57% |
|
~% |
|
~% |
|
~83% |