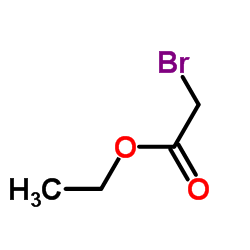
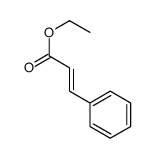
|
~69% |
|
~92% |
|
~70% |
|
~51% |
|
~41% |
|
~% |
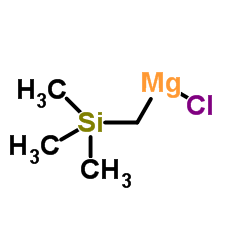
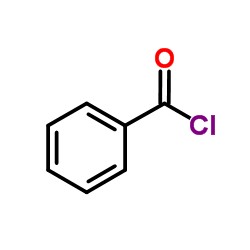
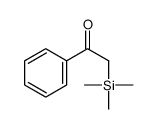
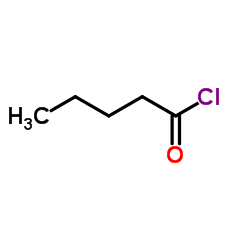



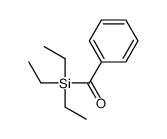
![[dimethyl(phenyl)silyl]-phenylmethanone结构式](https://image.chemsrc.com/caspic/214/17909-51-2.png)