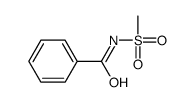
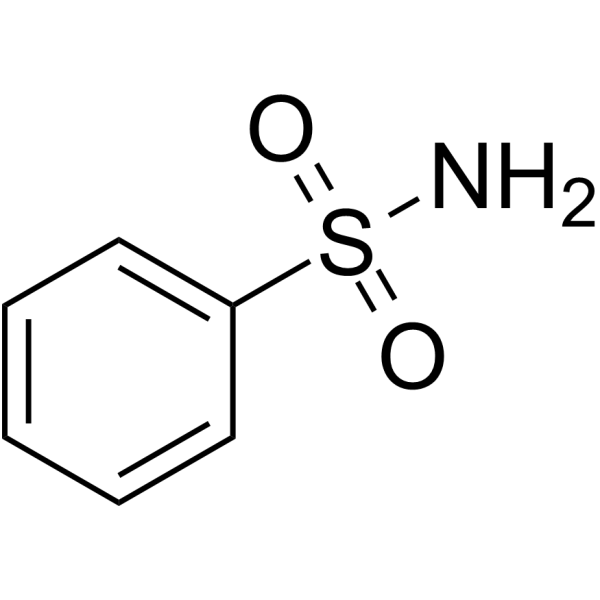
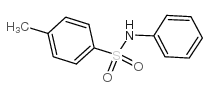
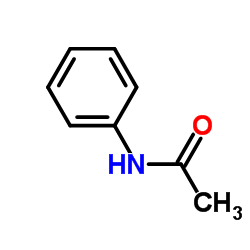
|
~96% |
|
~93% |
|
~69% |
|
~61% |
|
~77% |
|
~73% |
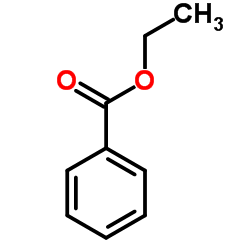
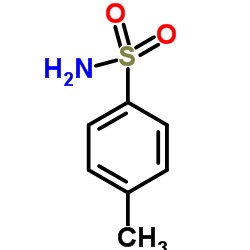
![Benzamide,N-[(4-methylphenyl)sulfonyl] Structure](https://image.chemsrc.com/caspic/260/6971-74-0.png)